Summary
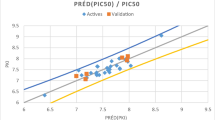
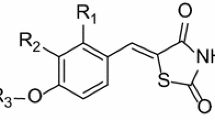
Quantitative structure-activity analysis was carried out for in vitro inhibition of rat brain soluble catechol O-methyltransferase by a series (N=99) of 1,5-substituted-3,4-dihydroxybenzenes using computational chemistry and multivariate PLS modelling of data sets. The molecular structural descriptors (N=19) associated with the electronics of the catecholic ring and sizes of substituents were derived theoretically. For the whole set of molecules two separate PLS models have to be used. A PLS model with two significant (crossvalidated) model dimensions describing 82.2% of the variance in inhibition activity data was capable of predicting all molecules except those having the largest R1 substituent or having a large R5 substituent compared to the NO2 group. The other PLS model with three significant (crossvalidated) model dimensions described 83.3% of the variance in inhibition activity data. This model could not handle compounds having a small R5 substituent, compared to the NO2 group, or the largest R1 substituent. The predictive capability of these PLS models was good. The models reveal that inhibition activity is nonlinearly related to the size of the R5 substituent. The analysis of the PLS models also shows that the binding affinity is greatly dependent on the electronic nature of both R1 and R5 substituents. The electron-withdrawing nature of the substituents enhances inhibition activity. In addition, the size of the R1 substituent and its lipophilicity are important in the binding of inhibitors. The size of the R1 substituent has an upper limit. On the other hand, ionized R1 substituents decrease inhibition activity.
Similar content being viewed by others
References
Axelrod, J. and Tomchick, R., J. Biol. Chem., 233 (1958) 702.
Guldberg, H. and Marsden, C., Pharmacol. Rev., 27 (1975) 135.
Ball, P., Knuppen, R., Haupt, M. and Breuer, H., J. Clin. Endocrinol., 34 (1972) 736.
Borchardt, R.T., In Jakoby, W.B. (Ed.), Enzymatic Basis of Detoxification, Vol. II, Academic Press, New York, 1980, p. 43.
Assicot, M. and Bohuon, C., Biochimie, 52 (1971) 871.
Nissinen, E., Biochem. Pharmacol., 33 (1984) 3105.
Salminen, M., Lundström, K., Tilgmann, C., Savolainen, R., Kalkkinen, N. and Ulmanen, I., Gene, 93 (1990) 241.
Tilgmann, C. and Kalkkinen, N., FEBS Lett., 264 (1990) 95.
Bertocci, B., Miggiano, V., DaPrada, M., Dembic, Z., Lahm, H.-W. and Malherbe, P., Proc. Natl. Acad. Sci. USA, 88 (1991) 1416.
Lundström, K., Salminen, M., Jalanko, A., Savolainen, R. and Ulmanen, I., DNA Cell Biol., 10 (1991) 181.
Linden, I.-B., Nissinen, E., Etemadzadeh, E., Kaakkola, S., Männistö, P. and Pohto, P., J. Pharmacol. Exp. Ther., 247 (1988) 289.
Männistö, P. and Kaakkola, S., Trends Pharmacol. Sci., 10 (1989) 54.
Bäckström, R., Honkanen, E., Pippuri, A., Kairisalo, P., Pystynen, J., Heinola, K., Nissinen, E., Linden, I.-B., Männistö, P., Kaakkola, S. and Pohto, P., J. Med. Chem., 32 (1989) 841.
Taskinen, J., Vidgren, J., Ovaska, M., Bäckström, R., Pippuri, A. and Nissinen, E., Quant. Struct.-Act. Relat., 8 (1989) 210.
DunnIII, W.J., Wold, S., Edlund, U., Hellberg, S. and Gasteiger, J., Quant. Struct.-Act. Relat., 3 (1984) 131.
Hellberg, S., Wold, S., DunnIII, W.J., Gasteiger, J. and Hutchings, M.G., Quant. Struct.-Act. Relat., 4 (1985) 1.
Wold, S., Albano, C., DunnIII, W.J., Edlund, U., Esbensen, K., Geladi, P., Hellberg, S., Johansson, E., Lindberg, W. and Sjöström, M., In Kowalski, B.R. (Ed.), Chemometrics-Mathematics and Statistics in Chemistry, NATO ASI Series C No. 138, Reidel Publ. Co., Dordrecht, 1984, pp. 17–95.
Cruciani, G., Baroni, M., Bonelli, D., Clementi, S., Ebert, C. and Skagerberg, B., Quant. Struct.-Act. Relat., 9 (1990) 101.
Jolliffe, I.T., Principal Component Analysis, Springer, New York, 1986.
Dewar, M.J.S., Zoebisch, E.G., Eamonn, F.H. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
CHEM-X, developed and distributed by Chemical Design Ltd., Oxford, UK.
Daylight Chemical Information Systems Inc., MedChem Software, Release 3.54, 1990.
Lindgren, F., Eriksson, L., Hellberg, S., Jonsson, J., Sjöström, M. and Wold, S., Quant. Struct.-Act. Relat., 10 (1991) 36.
Wold, S., Technometrics, 20 (1978) 397.
CramerIII, R.D., Bunce, J.D., Patterson, D.E. and Frank, I.E., Quant. Struct.-Act. Relat., 7 (1988) 18.
Hellberg, S., Sjöström, M., Skagerberg, G. and Wold, S., J. Med. Chem., 30 (1987) 1126.
Cocchi, M., Menziani, M.C., Rastelli, G. and DeBenedetti, P.G., Quant. Struct.-Act. Relat., 9 (1990) 340.
SIMCA-R Manual, Multivariate Data Analysis version 4.2, 1990 (UMETRI AB, Sweden).
Massart, D.L., Vandeginste, B.G.M., Deming, S.N., Michotte, Y. and Kaufman, L., Chemometrics: A Textbook. Data Handling in Science and Technology, Vol. 2, Elsevier, Amsterdam, 1988, pp. 403–407.
Norinder, U., J. Comput.-Aided Mol. Design., 4 (1990) 381.
Wold, S. Kettaneh-Wold, N. and Skagerberg, B., Chemometrics Intel. Lab. Sys., 7 (1989) 53.
Frank, I.E., Chemometrics Intel. Lab. Sys., 8 (1990) 109.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lotta, T., Taskinen, J., Bäckström, R. et al. PLS modelling of structure—activity relationships of catechol O-methyltransferase inhibitors. J Computer-Aided Mol Des 6, 253–272 (1992). https://doi.org/10.1007/BF00123380
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00123380