Summary
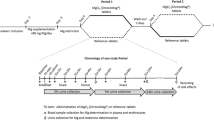
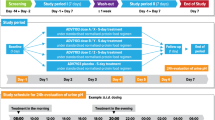
The relative availability of potassium from a controlled-release multiple-units tablet (Kalinorm) and a single-unit tablet (Slow-K) were compared in 13 volunteers on a low potassium diet (less than 30 mmol), by observing changes in urinary potassium excretion after administration of a single dose of 32 mmol potassium, either with or without water loading. Irrespective of procedure, the two products had the same extent of availability. The use of water loading, and special precautions about the level of dietary potassium and its composition when studying urinary potassium excretion, are discussed. It is suggested that water loading should be avoided when investigating the rate of potassium excretion.
Similar content being viewed by others
References
Bechgaard H, Hegermann Nielsen G, Aggerbeck A (1979) Kalinorm. En polydepot tablet med kontrolleret udløsning af kaliumklorid. In vitro og in vivo dokumentation. Farm Tid 89: 761–766
Ben-Ishay D, Engelman K (1973) Bioavailability of potassium from a slow-release tablet. Clin Pharmacol Ther 14: 250–258
Block BP, Thomas MB (1978) (Suppl) A method for testing intestinal irritancy of sustained release potassium chloride preparations in animals. J Pharm Pharmacol 30: 70 P
Farquharson-Roberts MA, Giddings AEB, Nunn AJ (1975) Perforation of small bowel due to slow release potassium chloride (Slow-K). Br Med J 3: 206
Goble FC, Lillis WG, Baldridge J, Jacob JT, Casorso DR (1980) Evaluation of intestinal ulcerogenesis potential of potassium chloride solid dosage forms. Curr Ther Res 27: 40–54
Howie AD, Strachan RW (1975) Slow release potassium chloride treatment. Br Med J 2: 176
McAvoy BR (1974) Mouth ulceration and slow-release potassium tablets. Br Med J 4: 164–165
McCall AJ (1975) Slow-K ulceration of oesophagus with aneurysmal left atrium. Br Med J 3: 230–231
McMahon FG, Akdamar K (1976) Gastric ulceration after “Slow-K”. N Engl J Med 295: 733–734
Pemberton J (1970) Oesophgeal obstruction and ulceration caused by oral potassium therapy. Br Heart J 32: 267–268
Renker von H, Schaub E, Bürgin M, Zanoletti A, Chovan K, Müller A (1977) Experimental study on the tolerance of different oral potassium preparations in rats. Arzneim Forsch (Drug Res) 27: 845–851
Skoutakis VA, Acchiardo SR, Feigenbaum AS (1979) Bioavailability of potassium from a slow-release tablet. Curr Ther Res 25: 104–112
Tannen RI., Cordano A (1978) Pharmakokinetics and effects on fecal blood loss of a controlled release potassium chloride tablet. J Pharmacol Exp Ther 204: 240–246
Weiss SM, Rutenberg HL, Paskin DL, Zaren HA (1977) Gut lesions due to slow-release KCl tablets. N Engl J Med 296: 111–112
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bechgaard, H., Shephard, N.W. Bioavailability of potassium from controlled-release tablets with and without water loading. Eur J Clin Pharmacol 21, 143–147 (1981). https://doi.org/10.1007/BF00637515
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00637515