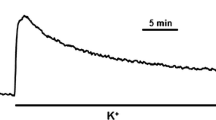
Abstract
This synopsis focuses on the role that tyrosine kinase pathways may play in the actue regulation of smooth muscle contractility by receptor-kinase-activating growth factors, such as epidermal growth factor-urogastrone (EGF-URO) and by G-protein-coupled agonists, such as angiotensin-II. Growth factor-activated response paradigms that modulate smooth muscle contractility are summarized and the parallels between the actions of G-protein-coupled agonists and growth factors in these response systems are pointed out. A possible dynamic interplay between tyrosine kinase and tyrosine phosphatase activities to modulate tissue tension is also hypothesized. Finally, a model is proposed, wherein an intermediary tyrosine kinase pathway is suggested as a point of convergence for the regulation of smooth muscle contractility by agonists as diverse as EGF-URO and angiotensin-II.
Similar content being viewed by others
References
Collett MS, Erikson RL: Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Science USA 75: 2021–2024, 1978
Hunter T, Sefton BM: Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA 77: 1311–1315, 1980
Hanks SK, Quinn AM, Hunter T: The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52, 1988
Courtneidge SA, Fumagalli S, Koegl M, Superti-Furga G, Twamley-Stein GM: The src family of protein tyrosine kinases: regulation and functions. Development Suppl: 57–64, 1993
Cooper JA: The src-family of protein-tyrosine kinases. In: Peptides and Protein Phosphorylation. B. Kemp and P.F. Alewood, (eds), CRC Press, Boca Raton, 1990, pp 85–113
Glenney JR, Jr: Tyrosine-phosphorylated proteins: mediators of signal transduction from the tyrosine kinases. Biochim Biophys Acta 1134: 113–127, 1992
Schlessinger J, Ullrich A: Growth factor signalling by receptor tyrosine kinases. Neuron 9: 383–391, 1992
Miyajima A, Kitamura T, Harada N, Yokota T, Arai K-I: Cytokine receptors and signal transduction. Ann Rev Immunol 10: 295–331, 1992
Kishimoto T, Taga T, Akira S: Cytokine signal transduction. Cell 76: 253–262, 1994
Hollenberg MD: The acute actions of growth factors in smooth muscle systems. Life Sci 54: 223–235, 1994
Muramatsu I, Hollenberg MD, Lederis K: Vascular actions of epidermal growth factor-urogastrone: possible relationships to prostaglandin production. Can J Physiol Pharmacol 63: 994–999, 1985
Muramatsu I, Itoh H, Lederis K, Hollenberg MD: Distinctive actions of epidermal growth factor-urogastrone in isolated smooth muscle preparations from guinea pig stomach: differential inhibition by indomethacin. J Pharmacol Exp Ther 245: 625–631, 1988
Patel P, Itoh H, Lederis K, Hollenberg MD: Contraction of guinea pig trachea by epidermal growth factor-urogastrone. Can J Physiol Pharmacol 66: 1308–1312, 1988
Gan BS, Hollenberg MD: Distinct coronary artery receptor systems for epidermal growth factor-urogastrone. J Pharmacol Exp Ther 252: 1277–1282, 1990
Yang S-G, Saifeddine M, Hollenberg MD: Tyrosine kinase inhibitors and the contractile action of epidermal growth factor-urogastrone and other agonists in gastric smooth muscle. Can J Physiol Pharmacol 70: 85–93, 1992
Yang S-G, Saifeddine M, Chuang M, Severson DL, Hollenberg MD: Diacylglycerol lipase and the contractile action of epidermal growth factor-urogastrone: evidence for distinct signal pathways in a single strip of gastric smooth muscle. Eur J Pharmacol Molec Pharmacol 207: 225–230, 1991
Bourgoin S, Grinstein S: Peroxides of vanadate induce activation of phospholipase D in HL-60 cells. J Biol Chem 267: 11908–11916, 1992
Uings IJ, Thompson NT, Randall RW, Spacey GD, Bonser RW, Hudson AT, Garland LG: Tyrosine phosphorylation is involved in receptor coupling to phospholipase D but not phospholipase C in the human neutrophil. Biochem J 281: 597–600, 1992
Dubyak GR, Schomisch SJ, Kusner DJ, Xie M: Phospholipase D activity in phagocytic leucocytes is synergistically regulated by G-protein- and tyrosine kinase-based mechanisms. Biochem J 292: 121–128, 1993.
Wilkes LC, Patel V, Purkiss JR, Boarder MR: Endothelin-1 stimulated phospholipase D in A10 vascular smooth muscle derived cells is dependent on tyrosine kinase. FEBS Lett 322: 147–150, 1993
Boarder MR: A role for phospholipase D in control of mitogenesis. Trends Pharmacol Sci 15: 57–62, 1994
Lee K-M, Toscas K, Villereal ML: Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J Biol Chem 268: 9945–9948, 1993
Muramatsu I, Hollenberg MD, Lederis K: Modulation by epidermal growth factor-urogastrone of contraction in isolated canine helical mesenteric arterial strips. Can J Physiol Pharmacol 64: 1561–1565, 1986
Carter NB, Fawcett AA, Hales JRS Moore GPM, Panaretto, BA: Circulatory effects of a depilatory dose of mouse epidermal growth factor in sheep. J Physiol (Lond) 403: 27–39, 1988
Scoggins BA, Butkus A, Coghlan JP, Fei DTW, McDougall JG, Niall HD, Wang X-M: In-vivo cardiovascular, renal and endocrine effects of epiderrnal growth factor in sheep. Abstr S-110, 7th Congr Endocrinol, Exerpta Medica Aust., 1984, p 124
Rodbell M: The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature 284: 17–22, 1980
Hepler JR, Gilman AG: G-proteins. Trends in Biol Sci 17: 383–387, 1992
DeVivo M, Iengar R: G-protein pathways: signal processing by effectors. Mol Cell Endocrinol 100: 65–70, 1994
Milligan G: Mechanisms of multifunctional signalling by G protein-linked receptors. Trends in Pharmacol Sci 14: 239–244, 1993
Levitzki A, Bar-Sinai A: The regulation of adenylyl cyclase by receptor-operated G proteins. Pharmacol Ther 50: 271–283, 1991
Levitzki A, Marbach I, Bar-Sinai A: The signal transduction between β-receptors and adenylyl cyclase. Life Sciences 52: 2093–2100, 1993
Birnbaumer L: G proteins in signal transduction. Annu Rev Pharmacol Toxicol 30: 675–705, 1990
Simon MI, Strathmann MP, Gautam N: Diversity of G proteins in signal transduction. Science 252: 802–808, 1991
Clapham DE, Neer EJ: New roles for G-protein βγ-dimers in transmembrane signalling. Nature 365: 403–406, 1993
Exton JH: Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta 1212: 26–42, 1994
Bourne HR, Sanders DA, McCormick F: The GTPase superfamily: conserved structure and molecular mechanism. Nature 349: 117–126, 1991
Berridge M: Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993
Huckle WR, Prokop CA, Dy RC, Herman B, Earp S: Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Molec Cell Biol 10: 6290–6298, 1990
Huckle WR, Dy RC, Earp HS Calcium-dependent increase in tyrosine kinase activity stimulated by angiotensin II. Proc Natl Acad Sci USA 89: 8837–8841, 1992
Force T, Kyriakis JA, Bonventre JV: Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and-independent pathways in glomerular mesangial cells. J Biol Chem 266: 6650–6656, 1991
Tsuda T, Kawahara Y, Shii K, Koide M, Ishida Y, Yokoyama M: Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett 285: 44–48, 1991
Zachary I, Gil J, Lehmann W, Sinnett-Smith J, Rozengurt E: Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swill 3T3 cells. Proc Natl Acad Sci USA 88: 4577–4581, 1991
Leeb-Lundberg LMF, Song X-H: Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem 266: 7746–7749, 1991
Molloy CJ, Taylor DS, Weber H: Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem 268: 7338–7345, 1993
Yang S-G, Saifeddine M, Laniyonu AA, Hollenberg MD: Distinct signal transduction pathways for angiotensin-II in guinea pig gastric smooth muscle: differential blockade by indomethacin and tyrosine kinase inhibitors. J Pharmacol Exp Ther 264: 958–966, 1993
Gazit A, Yaish P, Gilon C, Levitzki, A: Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem 32: 2344–2345, 1989
Levitzki A: Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J 6: 3275–3282, 1992
Laniyonu AA, Saifeddine M, Yang S-G, Hollenberg MD: Tyrosine kinase inhibitors and the contractile action of G-protein-linked vascular agonists. Can J Physiol Pharmacol 72: 1075–1085, 1994
Di Salvo J, Steusloff A, Semenchuk L, Satoh, S., Kolquist, K, Pfitzer G: Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun 190: 968–974, 1993
Griendling KK, Alexander RW: Angiotensin, other pressors and the transduction of vascular smooth muscle contraction. In: J.H. Laragh and B.M. Brenner (eds). Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press, New York, 1990, 1: pp 583–600
Kadota S, Fantus IG, Deragon G, Guyda HJ, Hersh B, Posner BI: Peroxide(s) of vanadium: a novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem Biophys Res Commun 147: 259–266, 1987
Fantus IG, Kadota S, Deragon G, Foster B, Posner BI: Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochem 28: 8864–8871, 1989
Laniyonu A, Saifeddine M, Ahmad S, Hollenberg MD: Regulation of vascular and gastric smooth muscle contractility by pervanadate. Brit J Pharmacol 113: 403–410, 1994
Heffetz D, Bushkin I, Dror R, Zick Y: The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem 265: 2896–2902, 1990
Takahasi K, Bardhan S, Kambayashi Y, Shirai H, Inagami T: Protein tyrosine phosphatase inhibition by angiotensin II in rat pheochromocytoma cells through type 2 receptor AT2. Biochem Biophys Res Commun 198: 60–66, 1994
Buscail L, Delesque N, Estève J-P, Saint-Laurent N, Prats H, Clerc P, Robberecht P, Bell GI, Liebow C, Schally AV, Vaysse N, Susni C: Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Natl Acad Sci USA 91: 2315–2319, 1994
Butcher RD, Schöllmann C, Marmé D: Angiotensin II mediates intracellular signalling in vascular smooth muscle cells by activation of tyrosine-specific protein kinases and C-RAF-1. Biochem Biophys Res Commun 196: 1280–1287, 1993
Earp HS, Huckle, WR: Intracellular calcium and the regulation of agonist-stimulated tyrosine kinase activity. Can J Physiol Pharmacol 1994, 72, Suppl. 1, 39
Zachary I, Rozengurt E: Focal Adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell 71: 891–894, 1992
Sinnett-Smith J, Zachary I, Valverde AM, Rozengurt E: Bombesin stimulation of p125 focal adhesion kinase tyrosine phosphorylation. J Biol Chem 268: 14261–14268, 1993
Zachary I, Sinnett-Smith J, Turner CE, Rozengurt E: Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation of the focal adhesion-associated protein paxillin in Swiss 3T3 cells. J Biol Chem 268: 22060–22065, 1993
Rozengurt E, Sinnett-Smith J, Zachary I, Seckl M, Rankin S: Agonist stimulation of tyrosine phosphorylation in cultured cell systems. Can J Physiol Pharmacol 1994, 72: Suppl., p 39
Cuatrecasas P, Hollenberg MD: Membrane receptors and hormone action. Adv Protein Chem 30: 251–451, 1976
Hollenberg MD: Tyrosine kinase pathways and the regulation of smooth muscle contractility. Trends in Pharmacol Sci 15: 108–114, 1994
Hernandez-Sotomayor SMT, Carpenter G: Epidermal growth factor receptor: elements of intracellular communication. J Membrane Biol 128: 81–89, 1992
Pawson T, Gish GD: SH2 and SH3 domains: from structure to function. Cell 71: 359–362, 1992
Courtneidge SA, Dhand R, Pilat D, Twamley GM, Waterfield MD, Roussel MF: Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J 12: 943–950, 1993
Mori S, Monnstrand L, Yokote K, Engstrom A, Courtneidge SA, Claesson-Welsh L, Heldin CH: Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J 12: 2257–2264, 1993
Velazquez L, Fellous M, Stark GR, Pellegrini S: A protein tyrosine kinase in the interferon α/β signalling pathway. Cell 70: 313–322, 1992
Shual K, Zlemlecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE: Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature 366: 580–583, 1993
Silvennolnen O, Ihle Schlessinger J, Levy DE: Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature 366: 583–585, 1993
Wang L-M, Keegan AD, Li W, Lienhard GE, Pacini S, Gutkind JS, Myers MR, Jr, Sun X-J, White MF, Aaronson SA, Paul WE, Pierce JH: Common elements in interleukin 4 and insulin signalling pathways in factor-dependent hematopoietic cells. Proc Natl Acad Sci USA 90: 4032–4036, 1993
Wijetunge S, Aalkjaer C, Schacter M, Hughes AD: Tyrosine kinase inhibitors block calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Commun 189: 1620–1623, 1992
Sargeant P, Farndale RW, Sage SO: The tyrosine kinase inhibitors methyl 2, 5-dihydroxycinnamate and genistein reduce thrombinevoked tyrosine phosphorylation and Ca2+ entry in human platelets. FEBS Lett 315: 242–246, 1993
Sargeant P, Farndale RW, Sage SO: ADP-and thapsigargin-evoked Ca2+ entry and protein-tyrosine phosphorylation are inhibited by the tyrosine kinase inhibitors genistein and methyl-2, 5-dihydroxycinnamate in fura-2-loaded human platelets
Gnegy ME: Calmodulin in neurotransmitter and hormone action. Ann Rev Pharmacol Tox 33: 45–70, 1993
Lu KP, Means AR: Regulation of the cell cycle by calcium and calmodulin. Endocrine Rev 14: 40–58, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hollenberg, M.D. Tyrosine kinase-mediated signal transduction pathways and the actions of polypeptide growth factors and G-protein-coupled agonists in smooth muscle. Mol Cell Biochem 149, 77–85 (1995). https://doi.org/10.1007/BF01076566
Issue Date:
DOI: https://doi.org/10.1007/BF01076566