Abstract
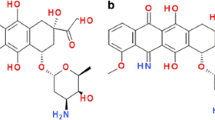
The ability of the cardioprotective agent ICRF-187 (dexrazoxane), its one-ring open hydrolysis products, and its two-ring open hydrolysis product, ADR-925, to displace Fe3+ from its complex with doxorubicin, daunorubicin, epirubicin and idarubicin have been studied. At pH 7.4, ICRF-187 at a concentration of 100 μM ICRF-187 slowly but completely displaced Fe3+ from its anthracycline complexes with half-times ranging from 230 to 450 min. The one-ring open intermediate hydrolysis products were also shown to be chelating agents and were also able to displace quickly and completely Fe3+ from its anthracycline complexes with half-times ranging from 1.7 to 16.7 min. Molecular modeling of Fe3+ complexes with the one-ring open intermediates showed that these intermediates were likely acting as tetradentate ligands. Since these intermediates are such good chelating agents, they may also be pharmacologically active species in preventing oxygen-radical derived iron-based anthracycline-induced cardiotoxicity. Since these one-ring open intermediates are produced by hydrolysis from the parent ICRF-187 more quickly than is ADR-925, the formation of pharmacologically active species might be occurring more quickly than previously thought. The displacement of Fe3+ from its anthracycline complexes by the two-ring open hydrolysis product ADR-925 also took place quickly and completely with half-times ranging from 1 to 3 min.
Similar content being viewed by others
References
J. L. Speyer, M. D. Green, E. Kramer, M. Rey, J. Sanger, C. Ward, N. Dubin, V. Ferrans, P. Stecy, A. Zeleniuch-Jacquotte, J. Wernz, F. Feit, W. Slater, R. Blum and F. Muggia,Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. New Engl. J. Med.319, 745–752 (1988).
J. L. Speyer, M. D. Green, A. Zeleniuch-Jacquotte, J. C. Wernz, M. Rey, J. Sanger, E. Kramer, V. Ferrans, H. Hochster, M. Meyers, R. H. Blum, F. Feit, M.Attubato, W. Burrows and F. M. Muggia,ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J. Clin. Oncol.10, 117–127 (1992).
J. Koning, P. Palmer, C. R. Franks, D. E. Mulder, J. L. Speyer, M. D. Green and K. Hellmann,Cardioxane-ICRF-187, towards anticancer drug specificity through selective toxicity reduction. Cancer Treat. Rev.18, 1–19 (1991).
E. H. Herman, V. J. Ferrans, C. E. Myers and J. F. Van Vleet,Comparison of the effectiveness of (±)-1,2-Bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and n-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res.45, 276–281 (1985).
E. H. Herman and V. J. Ferrans,Pretreatment with ICRF-187 provides long lasting protection against chronic daunorubicin cardiotoxicity in rabbits. Cancer Chemother. Pharmacol.16, 102–106 (1986).
P. M. Alderton, J. Gross and M. D. Green,Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res.52, 194–201 (1992).
E. H. Herman, A. El-Hage, Y. Fukada and V. J. Ferrans,ICRF-187 reduces bleomycin-induced pulmonary toxicity in mice. FASEB J.3, A275 (1989).
C. E. Myers, L. Gianni, C. B. Simone, R. Klecker and R. Greene,Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry21, 1707–1713 (1982).
E. J. F. Demant and P. K. Jensen,Destruction of phospholipids and respiratory-chain activity in pig-heart submitochondrial particles induced by an adriamycin-iron complex. Eur. J. Biochem.132, 551–556 (1983).
L. Gianni, B. J. Corden and C. E. Myers,The biochemical basis of anthracycline toxicity and anti-tumor activity. Rev. Biochem. Toxicol.5, 1–82 (1983).
J. M. C. Gutteridge,Lipid peroxidation and possible hydroxyl radical formation stimulated by the self-reduction of a doxorubicin-iron (III) complex. Biochem. Pharmacol.33, 1725–1728 (1984).
B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, pp. 489–492, Clarendon, Oxford 1989.
K. M. Dawson,Studies on the stability and cellular distribution of dioxopiperazines in cultured BHK-21S cells. Biochem. Pharmacol.24, 2249–2253 (1975).
B. B. Hasinoff,The interaction of the cardioprotective agent ICRF-187 ((+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane), its hydrolysis product ICRF-198, and other chelating agents with the Fe(III) and Cu(II) complexes of adriamycin. Agents and Actions26, 378–385 (1989).
B. B. Hasinoff,The iron(III)-adriamycin induced inactivation of the respiratory chain enzyme NADH cytochrome c reductase. Biochem. J.265, 865–870 (1990).
G. F. Vile and C. C. Winterbourn,dl-N,N′-dicarboxamidomethyl-N,N′-dicarboxymethyl-1,2-diaminopropane (ICRF-198) and d-1,2-bis(3,5-dioxopiperazine-1-yl)propane (ICRF-187) inhibition of Fe 3+ reduction, lipid peroxidation, and CaATPase inactivation in heart microsomes exposed to adriamycin. Cancer Res.50, 2307–2310 (1990).
D. D. Von Hoff, M. W. Lazard, P. Basa, H. L. Davis, A. L. Von Hoff, M. Rozencweig and F. Muggia,Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med.91, 710–717 (1979).
D. D. Von Hoff, M. Rozencweig, M. Layard, M. Slavik and F. M. Muggia,Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am. J. Med.62, 200–210 (1977).
T. G. Burke, T. D. Lee, J. van-Balgooy and J. H. Doroshow,Characterization of the aqueous decomposition products of (+) 1,2-bis(3,5-dioxopiperazinyl-1-yl)-propane (ICRF-187) by liquid chromatographic and mass spectral analysis. J. Pharm. Sci.80, 338–340 (1991).
B. B. Hasinoff,Pharmacodynamics of the hydrolysis-activation of the cardioprotective agent ICRF-187 ((+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane)). J. Pharm. Sci. (in press).
Z.-X. Huang, P. M. May, K. M. Quinlan, D. R. Williams and A. M. Creighton,Metal binding by pharmaceuticals. Part 2. Interactions of Ca(II), Cu(II), Fe(II), Mg(II), Mn(II) and Zn(II) with the intracellular hydrolysis products of the antitumor agent ICRF-159 and its inactive homologue ICRF-192. Agents and Actions12, 536–542 (1982).
B. B. Hasinoff, F. X. Reinders and V. Clark,The enzymatic hydrolysis-activation of the adriamycin cardioprotective agent (+)-1,2-bis(3,5-dioxopiperazinyl-1-yl) propane. Drug Metab. Dispos.19, 74–80 (1991).
P. M. May, G. K. Williams and D. R. Williams,Speciation studies of adriamycin, quelamycin, and their metal ion complexes. Inorg. Chim. Acta46, 221–228 (1980).
B. B. Hasinoff,The iron(III) and copper(II) complexes of adriamycin promote the hydrolysis of the cardioprotective agent ICRF-187 ((+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)-propane). Agents and Actions29, 374–381 (1990).
T. G. Burke, C. A. Pritos, A. C. Sartorelli and T. R. Tritton,The structural basis for anthracycline antibiotic stimulation of oxygen consumption by HL-60 cells and mitochondria. Cancer Biochem. Biophys.9, 245–255 (1987).
U. Burkert and N. L. Allinger, Molecular Mechanics. ACD Monograph 177, American Chemical Society, Washington 1982.
B. B. Hasinoff,Oxyradical production results from the Fe 3+-doxorubicin complex undergoing self-reduction by its α-ketol group. Biochem. Cell. Biol.68, 1331–1336 (1990).
B. B. Hasinoff,The hydrolysis-activation of the doxorubicin cardioprotective agent ICRF-187 ((+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane). Drug Metab. Dispos.18, 344–349 (1990).
C. K. Prout, D. S. Sanderson and M. C. Couldwell,N,N′-methylethylenebis (N-methylcarbamylglycinato) copper(II) dihydrate and N,N′-ethylethylenebis(N-methylcarbamylglycinato)-copper(II) dihydrate. Cryst. Struct. Comm.8, 181–188 (1979).
M. D. Lind, M. J. Hamor, T. A. Hamor and J. L. Hoard,Stereochemistry of ethylenediaminetetraacetato complexes. II. The structure of crystalline Rb[Fe(OH 2)Y·H 2 O]. III. The structure of crystalline Li[Fe(OH 2)Y·2H 2O]. Inorg. Chem.3, 34–43 (1964).
A. Hempel, N. Camerman and A. Camerman,Stereochemistry of the antitumor agent 4,4′-(1,2-propanediyl)bis(4-piperazine-2,6-dione): Crystal and molecular structure of the racemate (ICRF-159) and a soluble enantiomer (ICRF-187). J. Am. Chem. Soc.105, 3453–3456 (1982).
K. B. Nolan, T. Murphy, R. D. Hermanns and H. Rahoo,Chelating agents as antitumour drugs: Formation of chelating agents from the antitumor pro-drug razoxane. Inorg. Chim. Acta168, 283–288 (1990).
N. N. Daeid, K. B. Nolan and L. P. Ryan,Copper(II) complexes of hydrolysis products of the anticancer bis(3,5-dioxopiperazin-1-yl) alkanes. Displacement of co-ordinated carboxylate ligands by deprotonated amide groups in basic solution. J. Chem. Soc. Dalton Trans. 2301–2304 (1991).
M. M. Sobol, R. G. Amiet and M. D. Green,In vitro evidence for direct complexation of ADR-529/ICRF-187[(+)-1,2-bis-(3,5-dioxo-piperazin-1-yl) propane] onto an existing ferric-anthracycline complex. Mol. Pharmacol.41, 8–17 (1992).
B. B. Hasinoff and J. P. Davey,Adriamycin and its iron(III) and copper(II) complexes; glutathione-induced dissociation; cytochrome c oxidase inactivation and protection; binding to cardiolipin. Biochem. Pharmacol.37, 3663–3669 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buss, J.L., Hasinoff, B.B. The one-ring open hydrolysis product intermediates of the cardioprotective agent ICRF-187 (dexrazoxane) displace iron from iron-anthracycline complexes. Agents and Actions 40, 86–95 (1993). https://doi.org/10.1007/BF01976756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01976756