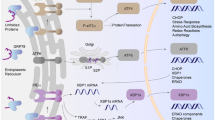
Abstract
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, accounting for 16.7 million deaths each year. The underlying cause of the majority of CVD is atherosclerosis. In the past, atherosclerosis was considered to be the result of passive lipid accumulation in the vessel wall. Today’s picture is far more complex. Atherosclerosis is considered a chronic inflammatory disease that results in the formation of plaques in large and mid-sized arteries. Both cells of the innate and the adaptive immune system play a crucial role in its pathogenesis. By transforming immune cells into pro- and anti-inflammatory chemokine- and cytokine-producing units, and by guiding the interactions between the different immune cells, the immune system decisively influences the propensity of a given plaque to rupture and cause clinical symptoms like myocardial infarction and stroke. In this review, we give an overview on the newest insights in the role of different immune cells and subtypes in atherosclerosis.


Similar content being viewed by others
References
World Health Organisation (2008) Global Health Observatory Data Repository—Mortality and burden of disease—WHO regions. WHO, Geneva
Mendis SP, Norrving, B (2011) Global Atlas on cardiovascular disease prevention and control. WHO, Geneva
Rosenfeld ME, Campbell LA (2011) Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost 106:858–867. doi:10.1160/TH11-06-0392
Sozeri B, Deveci M, Dincel N, Mir S (2012) The early cardiovascular changes in pediatric patients with systemic lupus erythematosus. Pediatr Nephrol. doi:10.1007/s00467-012-2342-2
de Leeuw K, Sanders JS, Stegeman C, Smit A, Kallenberg CG, Bijl M (2005) Accelerated atherosclerosis in patients with Wegener’s granulomatosis. Ann Rheum Dis 64:753–759. doi:10.1136/ard.2004.029033
Gonzalez-Gay MA, Szekanecz Z, Popa CD, Dessein P (2012) Atherosclerosis in rheumatoid arthritis. Mediat Inflamm 2012:489608. doi:10.1155/2012/489608
Lusis AJ (2000) Atherosclerosis. Nature 407:233–241. doi:10.1038/35025203
Lievens D, von Hundelshausen P (2011) Platelets in atherosclerosis. Thromb Haemost 106:827–838. doi:10.1160/TH11-08-0592
Koltsova EK, Ley K (2011) How dendritic cells shape atherosclerosis. Trends Immunol 32:540–547. doi:10.1016/j.it.2011.07.001
VanderLaan PA, Reardon CA, Getz GS (2004) Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24:12–22. doi:10.1161/01.ATV.0000105054.43931.f0
Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, Fievet C, Huc X, Barreira Y, Couloumiers JC, Arnal JF et al (2004) Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol 165:2013–2018
Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL (2004) Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci USA 101:17795–17800. doi:10.1073/pnas.0408096101
Woollard KJ, Geissmann F (2010) Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7:77–86. doi:10.1038/nrcardio.2009.228
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82
Weber C, Zernecke A, Libby P (2008) The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol 8:802–815. doi:10.1038/nri2415
Gautier EL, Jakubzick C, Randolph GJ (2009) Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol 29:1412–1418. doi:10.1161/ATVBAHA.108.180505
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ et al (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80. doi:10.1182/blood-2010-02-258558
Shantsila E, Lip GY (2009) Monocyte diversity in myocardial infarction. J Am Coll Cardiol 54:139–142. doi:10.1016/j.jacc.2009.03.047
Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW (2000) Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol 67:699–704
Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH (2011) SuperSAGE evidence for CD14++ CD16+ monocytes as a third monocyte subset. Blood 118:e50–e61. doi:10.1182/blood-2011-01-326827
Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ (2007) Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205. doi:10.1172/JCI29950
Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N et al (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117:185–194. doi:10.1172/JCI28549
Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N et al (2012) Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125:364–374. doi:10.1161/CIRCULATIONAHA.111.061986
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P et al (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616. doi:10.1126/science.1175202
Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C et al (2011) ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 121:4138–4149. doi:10.1172/JCI57559
Tall AR, Yvan-Charvet L, Westerterp M, Murphy AJ (2012) Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol 32:2547–2552. doi:10.1161/ATVBAHA.112.300134
Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649–1657. doi:10.1161/CIRCULATIONAHA.107.745091
Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R (2006) Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA 103:10340–10345. doi:10.1073/pnas.0604260103
Blanchet X, Langer M, Weber C, Koenen RR, von Hundelshausen P (2012) Touch of chemokines. Front Immunol 3:175. doi:10.3389/fimmu.2012.00175
Saederup N, Chan L, Lira SA, Charo IF (2008) Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117:1642–1648. doi:10.1161/CIRCULATIONAHA.107.743872
Boring L, Gosling J, Cleary M, Charo IF (1998) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394:894–897. doi:10.1038/29788
Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B et al (2007) Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol 27:373–379. doi:10.1161/01.ATV.0000253886.44609.ae
Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F (2004) Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res 94:253–261. doi:10.1161/01.RES.0000109793.17591.4E
Brito V, Mellal K, Portelance SG, Perez A, Soto Y, Deblois D, Ong H, Marleau S, Vazquez AM (2012) Induction of anti-anti-idiotype antibodies against sulfated glycosaminoglycans reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. doi:10.1161/ATVBAHA.112.300444
Cheng C, Tempel D, van Haperen R, de Boer HC, Segers D, Huisman M, van Zonneveld AJ, Leenen PJ, van der Steen A, Serruys PW et al (2007) Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J Clin Invest 117:616–626. doi:10.1172/JCI28180
Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ (2005) Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol 25:1014–1019. doi:10.1161/01.ATV.0000163181.40896.42
Aiello RJ, Perry BD, Bourassa PA, Robertson A, Weng W, Knight DR, Smith AH, Frederick KS, Kalgutkar A, Gladue RP (2010) CCR2 receptor blockade alters blood monocyte subpopulations but does not affect atherosclerotic lesions in apoE(−/−) mice. Atherosclerosis 208:370–375. doi:10.1016/j.atherosclerosis.2009.08.017
Mestas J, Ley K (2008) Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18:228–232. doi:10.1016/j.tcm.2008.11.004
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145:341–355. doi:10.1016/j.cell.2011.04.005
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15:551–561
Tabas I (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 10:36–46. doi:10.1038/nri2675
Burke-Gaffney A, Brooks AV, Bogle RG (2002) Regulation of chemokine expression in atherosclerosis. Vasc Pharmacol 38:283–292
Platt MO, Ankeny RF, Shi GP, Weiss D, Vega JD, Taylor WR, Jo H (2007) Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am J Physiol Heart Circ Physiol 292:H1479–H1486. doi:10.1152/ajpheart.00954.2006
Hennig T, Mogensen C, Kirsch J, Pohl U, Gloe T (2011) Shear stress induces the release of an endothelial elastase: role in integrin alpha(v)beta(3)-mediated FGF-2 release. J Vasc Res 48:453–464. doi:10.1159/000327009
Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD (2005) Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood 106:2334–2339. doi:10.1182/blood-2005-04-1530
Dong ZM, Brown AA, Wagner DD (2000) Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation 101:2290–2295
Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA (2012) Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res 110:889–900. doi:10.1161/CIRCRESAHA.111.263186
Eriksson EE (2011) Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation 124:2129–2138. doi:10.1161/CIRCULATIONAHA.111.030627
Johnson JL, Newby AC (2009) Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol 20:370–378. doi:10.1097/MOL.0b013e3283309848
Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M (1995) Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA 92:8264–8268
Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P et al (1997) Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol 150:1687–1699
Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M (2007) Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 100:884–893. doi:10.1161/01.RES.0000260802.75766.00
Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF (2000) Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol 20:2593–2599
Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T et al (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386:292–296. doi:10.1038/386292a0
Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, Gordon S, van der Laan LJ, Kraal G, Ishibashi S et al (1998) Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest 78:423–434
Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL (2000) Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105:1049–1056. doi:10.1172/JCI9259
Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW (2005) Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest 115:2192–2201. doi:10.1172/JCI24061
Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW (2009) Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol 29:19–26. doi:10.1161/ATVBAHA.108.176644
Brown MS, Ho YK, Goldstein JL (1980) The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem 255:9344–9352
Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N (2008) HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 7:365–375. doi:10.1016/j.cmet.2008.03.001
Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW et al (2010) ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328:1689–1693. doi:10.1126/science.1189731
Voloshyna I, Reiss AB (2011) The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res 50:213–224. doi:10.1016/j.plipres.2011.02.001
Seneviratne AN, Sivagurunathan B, Monaco C (2012) Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta 413:3–14. doi:10.1016/j.cca.2011.08.021
Liu G, Yang H (2012) Modulation of macrophage activation and programming in immunity. J Cell Physiol. doi:10.1002/jcp.24157
Kzhyshkowska J, Neyen C, Gordon S (2012) Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 217:492–502. doi:10.1016/j.imbio.2012.02.015
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi:10.1038/nri978
Tedgui A, Mallat Z (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86:515–581. doi:10.1152/physrev.00024.2005
Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y (2012) Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediat Inflamm 2012:693083. doi:10.1155/2012/693083
Wolfs IM, Donners MM, de Winther MP (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost 106:763–771. doi:10.1160/TH11-05-0320
Koenen RR, Weber C (2011) Chemokines: established and novel targets in atherosclerosis. EMBO Mol Med 3:713–725. doi:10.1002/emmm.201100183
Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27:165–197. doi:10.1146/annurev.immunol.021908.132620
de Jager SC, Bermudez B, Bot I, Koenen RR, Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJ, Weber C et al (2011) Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2-mediated macrophage chemotaxis. J Exp Med 208:217–225. doi:10.1084/jem.20100370
Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M (2002) Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol 168:1435–1440
Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL (2003) Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 278:1561–1568. doi:10.1074/jbc.M209634200
Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104:210–218, 221p following 218. doi: 10.1161/CIRCRESAHA.108.181040
Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J et al (2001) Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104:3103–3108
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M et al (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361. doi:10.1038/nature08938
Tabas I (2010) The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res 107:839–850. doi:10.1161/CIRCRESAHA.110.224766
Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab 9:474–481. doi:10.1016/j.cmet.2009.03.003
Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K et al (2010) The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol 30:1925–1932. doi:10.1161/ATVBAHA.110.206094
Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME et al (2009) Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119:2925–2941. doi:10.1172/JCI38857
Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG et al (2009) Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104:e42–e54. doi:10.1161/CIRCRESAHA.108.188771
Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I (2008) Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation 117:940–951. doi:10.1161/CIRCULATIONAHA.107.711275
Skjot-Arkil H, Barascuk N, Register T, Karsdal MA (2010) Macrophage-mediated proteolytic remodeling of the extracellular matrix in atherosclerosis results in neoepitopes: a potential new class of biochemical markers. Assay Drug Dev Technol 8:542–552. doi:10.1089/adt.2009.0258
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604. doi:10.1016/j.immuni.2010.05.007
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG (2011) Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22:317–326. doi:10.1681/ASN.2009060615
Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N et al (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143. doi:10.1016/j.cmet.2007.06.010
Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP (2012) Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225:461–468. doi:10.1016/j.atherosclerosis.2012.09.013
Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A et al (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res 108:985–995. doi:10.1161/CIRCRESAHA.110.233775
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311
Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C (2012) Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem 287:11629–11641. doi:10.1074/jbc.M111.338673
Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A et al (2010) Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5:e8852. doi:10.1371/journal.pone.0008852
Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA (2011) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123:989–998. doi:10.1161/CIRCULATIONAHA.110.984146
La Flamme AC, Harvie M, Kenwright D, Cameron K, Rawlence N, Low YS, McKenzie S (2007) Chronic exposure to schistosome eggs reduces serum cholesterol but has no effect on atherosclerotic lesion development. Parasite Immunol 29:259–266. doi:10.1111/j.1365-3024.2007.00942.x
Doenhoff MJ, Stanley RG, Griffiths K, Jackson CL (2002) An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology 125:415–421
Stanley RG, Jackson CL, Griffiths K, Doenhoff MJ (2009) Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis 207:131–138. doi:10.1016/j.atherosclerosis.2009.04.037
Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W et al (2010) Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107:737–746. doi:10.1161/CIRCRESAHA.109.215715
Gleissner CA, Shaked I, Little KM, Ley K (2010) CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol 184:4810–4818. doi:10.4049/jimmunol.0901368
Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, Vink A, de Kleijn DP (2010) High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 30:1842–1848. doi:10.1161/ATVBAHA.110.209296
Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O (2010) Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122:1837–1845. doi:10.1161/CIRCULATIONAHA.110.961714
Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Bjorkstrom NK, Malmberg KJ, Lindbom L, Eriksson EE (2010) Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am J Pathol 177:493–500. doi:10.2353/ajpath.2010.090480
Rotzius P, Soehnlein O, Kenne E, Lindbom L, Nystrom K, Thams S, Eriksson EE (2009) ApoE(−/−)/lysozyme M(EGFP/EGFP) mice as a versatile model to study monocyte and neutrophil trafficking in atherosclerosis. Atherosclerosis 202:111–118. doi:10.1016/j.atherosclerosis.2008.04.009
van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW (2008) Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol 28:84–89. doi:10.1161/ATVBAHA.107.154807
Daugherty A, Dunn JL, Rateri DL, Heinecke JW (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94:437–444. doi:10.1172/JCI117342
Lee TD, Gonzalez ML, Kumar P, Chary-Reddy S, Grammas P, Pereira HA (2002) CAP37, a novel inflammatory mediator: its expression in endothelial cells and localization to atherosclerotic lesions. Am J Pathol 160:841–848. doi:10.1016/S0002-9440(10)64907-3
Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, Xu Q, Yan ZQ (2006) Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol 26:1551–1557. doi:10.1161/01.ATV.0000223901.08459.57
Barnathan ES, Raghunath PN, Tomaszewski JE, Ganz T, Cines DB, Higazi Aa-R (1997) Immunohistochemical localization of defensin in human coronary vessels. Am J Pathol 150:1009–1020
Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK (2006) Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol 26:136–142. doi:10.1161/01.ATV.0000193567.88685.f4
Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O (2012) Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res 110:1052–1056. doi:10.1161/CIRCRESAHA.112.265868
Higazi AA, Lavi E, Bdeir K, Ulrich AM, Jamieson DG, Rader DJ, Usher DC, Kane W, Ganz T, Cines DB (1997) Defensin stimulates the binding of lipoprotein (a) to human vascular endothelial and smooth muscle cells. Blood 89:4290–4298
Bdeir K, Cane W, Canziani G, Chaiken I, Weisel J, Koschinsky ML, Lawn RM, Bannerman PG, Sachais BS, Kuo A et al (1999) Defensin promotes the binding of lipoprotein(a) to vascular matrix. Blood 94:2007–2019
Soehnlein O (2012) Multiple roles for neutrophils in atherosclerosis. Circ Res 110:875–888. doi:10.1161/CIRCRESAHA.111.257535
Dorweiler B, Torzewski M, Dahm M, Kirkpatrick CJ, Lackner KJ, Vahl CF (2008) Subendothelial infiltration of neutrophil granulocytes and liberation of matrix-destabilizing enzymes in an experimental model of human neo-intima. Thromb Haemost 99:373–381. doi:10.1160/TH07-06-0387
Belz GT, Nutt SL (2012) Transcriptional programming of the dendritic cell network. Nat Rev Immunol 12:101–113. doi:10.1038/nri3149
Becker L, Liu NC, Averill MM, Yuan W, Pamir N, Peng Y, Irwin AD, Fu X, Bornfeldt KE, Heinecke JW (2012) Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS ONE 7:e33297. doi:10.1371/journal.pone.0033297
Ley K, Miller YI, Hedrick CC (2011) Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 31:1506–1516. doi:10.1161/ATVBAHA.110.221127
Steinman RM, Cohn ZA (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137:1142–1162
Bobryshev YV, Lord RS (1995) S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc Res 29:689–696
Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G (2001) Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol 21:503–508
Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T et al (2004) Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis 176:101–110. doi:10.1016/j.atherosclerosis.2004.04.027
Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM (2009) Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 206:497–505. doi:10.1084/jem.20082129
Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K (2006) Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med 203:1273–1282. doi:10.1084/jem.20052205
Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E et al (2011) CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest 121:2898–2910. doi:10.1172/JCI44925
Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD (2008) CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28:243–250. doi:10.1161/ATVBAHA.107.158675
Bobryshev YV (2005) Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur Heart J 26:1700–1704. doi:10.1093/eurheartj/ehi282
Erbel C, Sato K, Meyer FB, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2007) Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol 102:123–132. doi:10.1007/s00395-006-0636-x
Yilmaz A, Weber J, Cicha I, Stumpf C, Klein M, Raithel D, Daniel WG, Garlichs CD (2006) Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J Am Coll Cardiol 48:70–80. doi:10.1016/j.jacc.2006.01.078
Van Vre EA, Hoymans VY, Bult H, Lenjou M, Van Bockstaele DR, Vrints CJ, Bosmans JM (2006) Decreased number of circulating plasmacytoid dendritic cells in patients with atherosclerotic coronary artery disease. Coron Artery Dis 17:243–248
Van Vre EA, Van Brussel I, de Beeck KO, Hoymans VY, Vrints CJ, Bult H, Bosmans JM (2010) Changes in blood dendritic cell counts in relation to type of coronary artery disease and brachial endothelial cell function. Coron Artery Dis 21:87–96. doi:10.1097/MCA.0b013e3283368c0e
Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ et al (2009) Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 119:2367–2375. doi:10.1161/CIRCULATIONAHA.108.807537
Hjerpe C, Johansson D, Hermansson A, Hansson GK, Zhou X (2010) Dendritic cells pulsed with malondialdehyde modified low density lipoprotein aggravate atherosclerosis in Apoe(−/−) mice. Atherosclerosis 209:436–441. doi:10.1016/j.atherosclerosis.2009.10.003
Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK (2011) Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 123:1083–1091. doi:10.1161/CIRCULATIONAHA.110.973222
Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, van Berkel TJ, Toes RE, Kuiper J (2010) Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc Res 85:622–630. doi:10.1093/cvr/cvp338
Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C et al (2011) Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res 109:1387–1395. doi:10.1161/CIRCRESAHA.111.256529
Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J et al (2012) Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125:1673–1683. doi:10.1161/CIRCULATIONAHA.111.046755
Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM et al (2012) Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 32:2569–2579. doi:10.1161/ATVBAHA.112.251314
Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z (2003) Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107:1009–1016
Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI (2006) Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203:2073–2083. doi:10.1084/jem.20060245
Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI (2009) GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med 206:2141–2149. doi:10.1084/jem.20090866
Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG (2005) Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22:439–450. doi:10.1016/j.immuni.2005.02.007
Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH (2008) CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res 103:965–973. doi:10.1161/CIRCRESAHA.108.185793
Packard RR, Shi GP (2006) Atherosclerosis progression and monocyte emigration from plaque. Future Cardiol 2:415–418. doi:10.2217/14796678.2.4.415
Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI (2010) Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res 106:383–390. doi:10.1161/CIRCRESAHA.109.210781
Cho HJ, Shashkin P, Gleissner CA, Dunson D, Jain N, Lee JK, Miller Y, Ley K (2007) Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics 29:149–160. doi:10.1152/physiolgenomics.00051.2006
Alderman CJ, Bunyard PR, Chain BM, Foreman JC, Leake DS, Katz DR (2002) Effects of oxidised low density lipoprotein on dendritic cells: a possible immunoregulatory component of the atherogenic micro-environment? Cardiovasc Res 55:806–819
Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stockl J et al (2005) Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol 175:501–508
Boulesteix T, Pena AM, Pages N, Godeau G, Sauviat MP, Beaurepaire E, Schanne-Klein MC (2006) Micrometer scale ex vivo multiphoton imaging of unstained arterial wall structure. Cytometry A 69:20–26. doi:10.1002/cyto.a.20196
Perrin-Cocon L, Coutant F, Agaugue S, Deforges S, Andre P, Lotteau V (2001) Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J Immunol 167:3785–3791
Sun J, Hartvigsen K, Chou MY, Zhang Y, Sukhova GK, Zhang J, Lopez-Ilasaca M, Diehl CJ, Yakov N, Harats D et al (2010) Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation 122:808–820. doi:10.1161/CIRCULATIONAHA.109.891887
Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH (2004) B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation 109:2009–2015. doi:10.1161/01.CIR.0000127121.16815.F1
Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK (2010) Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med 207:1081–1093. doi:10.1084/jem.20092243
Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT et al (2011) Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35:819–831. doi:10.1016/j.immuni.2011.09.014
Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ (2004) Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA 101:11779–11784. doi:10.1073/pnas.0403259101
Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S et al (2004) Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 110:3493–3500. doi:10.1161/01.CIR.0000148135.08582.97
Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R (1999) Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 19:2364–2367
Davenport P, Tipping PG (2003) The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol 163:1117–1125. doi:10.1016/S0002-9440(10)63471-2
Lee TS, Yen HC, Pan CC, Chau LY (1999) The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19:734–742
Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J (2005) Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 112:1054–1062. doi:10.1161/CIRCULATIONAHA.104.533463
Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2006) Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res 98:524–531. doi:10.1161/01.RES.0000204452.46568.57
Samarasinghe R, Tailor P, Tamura T, Kaisho T, Akira S, Ozato K (2006) Induction of an anti-inflammatory cytokine, IL-10, in dendritic cells after toll-like receptor signaling. J Interferon Cytokine Res 26:893–900. doi:10.1089/jir.2006.26.893
Kastelein RA, Hunter CA, Cua DJ (2007) Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 25:221–242. doi:10.1146/annurev.immunol.22.012703.104758
Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ et al (2010) Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab 12:142–153. doi:10.1016/j.cmet.2010.06.008
Greaves DR, Hakkinen T, Lucas AD, Liddiard K, Jones E, Quinn CM, Senaratne J, Green FR, Tyson K, Boyle J et al (2001) Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus- and activation-regulated chemokine, are expressed in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 21:923–929
Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM (2001) Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 193:713–726
Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG (2001) B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol 2:1126–1132. doi:10.1038/ni735
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi:10.1146/annurev.immunol.18.1.767
Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM (2006) Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 114:2482–2489. doi:10.1161/CIRCULATIONAHA.106.642801
Niessner A, Weyand CM (2010) Dendritic cells in atherosclerotic disease. Clin Immunol 134:25–32. doi:10.1016/j.clim.2009.05.006
Zhu J, Paul WE (2010) Heterogeneity and plasticity of T helper cells. Cell Res 20:4–12. doi:10.1038/cr.2009.138
Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK (1985) Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest 76:125–131. doi:10.1172/JCI111934
Hansson GK, Jonasson L, Holm J, Claesson-Welsh L (1986) Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol 64:261–268
Hansson GK, Holm J, Jonasson L (1989) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–175
Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL (1989) Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA 86:1372–1376
Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 92:3893–3897
Paulsson G, Zhou X, Tornquist E, Hansson GK (2000) Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 20:10–17
Dansky HM, Charlton SA, Harper MM, Smith JD (1997) T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 94:4642–4646
Song L, Leung C, Schindler C (2001) Lymphocytes are important in early atherosclerosis. J Clin Invest 108:251–259. doi:10.1172/JCI11380
Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS (2003) Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vasc Biol 23:1449–1454. doi:10.1161/01.ATV.0000079793.58054.2E
Hansson GK, Robertson AK, Soderberg-Naucler C (2006) Inflammation and atherosclerosis. Annu Rev Pathol 1:297–329. doi:10.1146/annurev.pathol.1.110304.100100
Kovanen PT (2007) Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev 217:105–122. doi:10.1111/j.1600-065X.2007.00515.x
Huber SA, Sakkinen P, David C, Newell MK, Tracy RP (2001) T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 103:2610–2616
Emeson EE, Shen ML, Bell CG, Qureshi A (1996) Inhibition of atherosclerosis in CD4 T-cell-ablated and nude (nu/nu) C57BL/6 hyperlipidemic mice. Am J Pathol 149:675–685
Zhou X, Nicoletti A, Elhage R, Hansson GK (2000) Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102:2919–2922
Ludewig B, Freigang S, Jaggi M, Kurrer MO, Pei YC, Vlk L, Odermatt B, Zinkernagel RM, Hengartner H (2000) Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc Natl Acad Sci USA 97:12752–12757. doi:10.1073/pnas.220427097
Olofsson PS, Soderstrom LA, Wagsater D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P et al (2008) CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 117:1292–1301. doi:10.1161/CIRCULATIONAHA.107.699173
Bobryshev YV, Lord RS (1998) Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res 37:799–810
De Palma R, Del Galdo F, Abbate G, Chiariello M, Calabro R, Forte L, Cimmino G, Papa MF, Russo MG, Ambrosio G et al (2006) Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation 113:640–646. doi:10.1161/CIRCULATIONAHA.105.537712
Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM (2000) Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101:2883–2888
Leon ML, Zuckerman SH (2005) Gamma interferon: a central mediator in atherosclerosis. Inflamm Res 54:395–411. doi:10.1007/s00011-005-1377-2
Harvey EJ, Ramji DP (2005) Interferon-gamma and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res 67:11–20. doi:10.1016/j.cardiores.2005.04.019
Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G (1989) Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med 170:1595–1608
Amento EP, Ehsani N, Palmer H, Libby P (1991) Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb 11:1223–1230
McLaren JE, Ramji DP (2009) Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev 20:125–135. doi:10.1016/j.cytogfr.2008.11.003
Whitman SC, Ravisankar P, Daugherty A (2002) IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res 22:661–670. doi:10.1089/10799900260100141
Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C (1997) IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 99:2752–2761. doi:10.1172/JCI119465
Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH (2003) Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol 23:454–460. doi:10.1161/01.ATV.0000059419.11002.6E
Whitman SC, Ravisankar P, Elam H, Daugherty A (2000) Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E-/- mice. Am J Pathol 157:1819–1824
Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER (2005) Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol 45:1939–1945. doi:10.1016/j.jacc.2005.03.040
Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145:33–43
Zhao Z, Wu Y, Cheng M, Ji Y, Yang X, Liu P, Jia S, Yuan Z (2011) Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis 217:518–524. doi:10.1016/j.atherosclerosis.2011.03.043
Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U (2002) Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med 195:245–257
Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A (2001) Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 89:E41–E45
Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK (2003) Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res 59:234–240
Whitman SC, Ravisankar P, Daugherty A (2002) Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res 90:E34–E38
Tenger C, Sundborger A, Jawien J, Zhou X (2005) IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler Thromb Vasc Biol 25:791–796. doi:10.1161/01.ATV.0000153516.02782.65
Mallat Z, Taleb S, Ait-Oufella H, Tedgui A (2009) The role of adaptive T cell immunity in atherosclerosis. J Lipid Res 50(Suppl):S364–S369. doi:10.1194/jlr.R800092-JLR200
King VL, Cassis LA, Daugherty A (2007) Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol 171:2040–2047. doi:10.2353/ajpath.2007.060857
King VL, Szilvassy SJ, Daugherty A (2002) Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol 22:456–461
Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ (2012) Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med 4:1072–1086. doi:10.1002/emmm.201201374
Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY (2008) IL-33 reduces the development of atherosclerosis. J Exp Med 205:339–346. doi:10.1084/jem.20071868
Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 114:427–437. doi:10.1172/JCI20479
Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK et al (1999) Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 103:117–128. doi:10.1172/JCI4533
Veillard NR, Steffens S, Burger F, Pelli G, Mach F (2004) Differential expression patterns of proinflammatory and antiinflammatory mediators during atherogenesis in mice. Arterioscler Thromb Vasc Biol 24:2339–2344. doi:10.1161/01.ATV.0000146532.98235.e6
de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC (2007) Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE 2:e779. doi:10.1371/journal.pone.0000779
Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S et al (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12:178–180. doi:10.1038/nm1343
Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H (2003) Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 108:1232–1237. doi:10.1161/01.CIR.0000089083.61317.A1
Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F (2005) T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med 201:737–746. doi:10.1084/jem.20040685
Andersson J, Libby P, Hansson GK (2010) Adaptive immunity and atherosclerosis. Clin Immunol 134:33–46. doi:10.1016/j.clim.2009.07.002
Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R et al (2007) Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115:2168–2177. doi:10.1161/CIRCULATIONAHA.106.662080
Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G (2007) MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest 117:1902–1913. doi:10.1172/JCI30966
Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C et al (1999) Protective role of interleukin-10 in atherosclerosis. Circ Res 85:e17–e24
Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A (2003) Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 9:10–17
Milner JD, Sandler NG, Douek DC (2010) Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr Opin HIV AIDS 5:179–183. doi:10.1097/COH.0b013e328335ed3e
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8:1390–1397. doi:10.1038/ni1539
Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E (2010) Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121:1746–1755. doi:10.1161/CIRCULATIONAHA.109.924886
Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Bockler D, Katus HA, Gleissner CA (2011) Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol 106:125–134. doi:10.1007/s00395-010-0135-y
Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X et al (2010) The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine 49:185–193. doi:10.1016/j.cyto.2009.09.007
Lahoute C, Herbin O, Mallat Z, Tedgui A (2011) Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol 8:348–358. doi:10.1038/nrcardio.2011.62
van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J (2009) Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun 388:261–265. doi:10.1016/j.bbrc.2009.07.152
Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ (2009) Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 183:8167–8175. doi:10.4049/jimmunol.0901126
Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C et al (2010) A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol 185:5820–5827. doi:10.4049/jimmunol.1000116
Butcher MJ, Gjurich BN, Phillips T, Galkina EV (2012) The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res 110:675–687. doi:10.1161/CIRCRESAHA.111.261784
Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J et al (2009) Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med 206:2067–2077. doi:10.1084/jem.20090545
Roselaar SE, Kakkanathu PX, Daugherty A (1996) Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol 16:1013–1018
Gewaltig J, Kummer M, Koella C, Cathomas G, Biedermann BC (2008) Requirements for CD8 T-cell migration into the human arterial wall. Hum Pathol 39:1756–1762. doi:10.1016/j.humpath.2008.04.018
Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J (2010) CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe(−)(/)(−) mice. BMC Immunol 11:58. doi:10.1186/1471-2172-11-58
Braun NA, Covarrubias R, Major AS (2010) Natural killer T cells and atherosclerosis: form and function meet pathogenesis. J Innate Immun 2:316–324. doi:10.1159/000296915
Nowak M, Stein-Streilein J (2007) Invariant NKT cells and tolerance. Int Rev Immunol 26:95–119. doi:10.1080/08830180601070195
Bobryshev YV, Lord RS (2005) Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem 53:781–785. doi:10.1369/jhc.4B6570.2005
Major AS, Wilson MT, McCaleb JL, Ru SuY, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF (2004) Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 24:2351–2357. doi:10.1161/01.ATV.0000147112.84168.87
Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS et al (2004) Natural killer T cells accelerate atherogenesis in mice. Blood 104:2051–2059. doi:10.1182/blood-2003-10-3485
Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP (2004) CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med 199:417–422. doi:10.1084/jem.20030997
VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS (2007) Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol 170:1100–1107. doi:10.2353/ajpath.2007.060188
Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC (2008) Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res 78:167–174. doi:10.1093/cvr/cvn005
To K, Agrotis A, Besra G, Bobik A, Toh BH (2009) NKT cell subsets mediate differential proatherogenic effects in ApoE−/− mice. Arterioscler Thromb Vasc Biol 29:671–677. doi:10.1161/ATVBAHA.108.182592
Aslanian AM, Chapman HA, Charo IF (2005) Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 25:628–632. doi:10.1161/01.ATV.0000153046.59370.13
Kyaw T, Tipping P, Toh BH, Bobik A (2011) Current understanding of the role of B cell subsets and intimal and adventitial B cells in atherosclerosis. Curr Opin Lipidol 22:373–379. doi:10.1097/MOL.0b013e32834adaf3
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1:475–482. doi:10.1038/82717
van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE (1989) Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 61:166–170
Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL (1994) Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb 14:32–40
van Leeuwen M, Damoiseaux J, Duijvestijn A, Tervaert JW (2009) The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmun Rev 9:53–57. doi:10.1016/j.autrev.2009.03.001
Caligiuri G, Nicoletti A, Poirier B, Hansson GK (2002) Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109:745–753. doi:10.1172/JCI7272
Major AS, Fazio S, Linton MF (2002) B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 22:1892–1898
Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P et al (2010) Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol 185:4410–4419. doi:10.4049/jimmunol.1000033
Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vre E, Esposito B, Vilar J et al (2010) B cell depletion reduces the development of atherosclerosis in mice. J Exp Med 207:1579–1587. doi:10.1084/jem.20100155
Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, Toh BH (2012) Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS ONE 7:e29371. doi:10.1371/journal.pone.0029371
Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL (1996) Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 98:800–814. doi:10.1172/JCI118853
Tsimikas S (2006) Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep 8:55–61
Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB (2007) Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res 48:425–433. doi:10.1194/jlr.M600361-JLR200
Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL (1992) Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 339:883–887
Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL (1995) Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol 15:1569–1576
Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL (2000) Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 105:1731–1740. doi:10.1172/JCI8472
Nilsson J, Hansson GK, Shah PK (2005) Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler Thromb Vasc Biol 25:18–28. doi:10.1161/01.ATV.0000149142.42590.a2
Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ (2003) Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 9:736–743. doi:10.1038/nm876
Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO (2009) Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 120:417–426. doi:10.1161/CIRCULATIONAHA.109.868158
Hulthe J, Bokemark L, Fagerberg B (2001) Antibodies to oxidized LDL in relation to intima-media thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol 21:101–107
Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S (2003) Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108:2107–2112. doi:10.1161/01.CIR.0000092891.55157.A7
Mizoguchi A, Bhan AK (2006) A case for regulatory B cells. J Immunol 176:705–710
Gerdes N, Zirlik A (2011) Co-stimulatory molecules in and beyond co-stimulation—tipping the balance in atherosclerosis? Thromb Haemost 106:804–813. doi:10.1160/TH11-09-0605
Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S et al (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12:178–180
Afek A, Harats D, Roth A, Keren G, George J (2005) A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis 183:57–63. doi:10.1016/j.atherosclerosis.2005.03.040
Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH (2006) Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation 114:2047–2055. doi:10.1161/CIRCULATIONAHA.106.633263
Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH (2007) Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 117:2974–2982. doi:10.1172/JCI31344
Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samnegard A, Petros C, Rollins J, Bennet AM, Wiman B et al (2005) Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet 37:365–372
van Wanrooij EJ, van Puijvelde GH, de Vos P, Yagita H, van Berkel TJ, Kuiper J (2007) Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 27:204–210
Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P (1998) Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394:200–203. doi:10.1038/28204
Lutgens E, Gorelik L, Daemen MJ, de Muinck ED, Grewal IS, Koteliansky VE, Flavell RA (1999) Requirement for CD154 in the progression of atherosclerosis. Nat Med 5:1313–1316. doi:10.1038/15271
Lutgens E, Cleutjens KB, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJ (2000) Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc Natl Acad Sci USA 97:7464–7469
Schonbeck U, Sukhova GK, Shimizu K, Mach F, Libby P (2000) Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci USA 97:7458–7463
Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM et al (2010) Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med 207:391–404. doi:10.1084/jem.20091293
Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, Ahrens I, Ernst S, Bassler N, Missiou A et al (2007) CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 115:1571–1580. doi:10.1161/CIRCULATIONAHA.106.683201
Bavendiek U, Zirlik A, LaClair S, MacFarlane L, Libby P, Schonbeck U (2005) Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler Thromb Vasc Biol 25:1244–1249. doi:10.1161/01.ATV.0000161420.55482.ef
Smook ML, Heeringa P, Damoiseaux JG, Daemen MJ, de Winther MP, Gijbels MJ, Beckers L, Lutgens E, Tervaert JW (2005) Leukocyte CD40L deficiency affects the CD25(+) CD4 T cell population but does not affect atherosclerosis. Atherosclerosis 183:275–282. doi:10.1016/j.atherosclerosis.2005.03.051
Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, van Kruchten R, Thevissen L et al (2010) Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 116:4317–4327. doi:10.1182/blood-2010-01-261206
Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, Wijnands E, Cleutjens J, Zernecke A, Weber C et al (2008) The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood 111:4596–4604. doi:10.1182/blood-2007-05-088906
Acknowledgments
This work was supported by the AMC fellowship grant (E.L.), Humboldt Foundation (Sofja Kovalevskaja grant to E.L.), the Netherlands Organization for Scientific Research (VIDI grant to E.L.), the Netherlands Heart Foundation (established investigator grant to E.L.), and the Deutsche Forschungsgemeinschaft (DFG FOR809 and SFB 1054 to E.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Legein, B., Temmerman, L., Biessen, E.A.L. et al. Inflammation and immune system interactions in atherosclerosis. Cell. Mol. Life Sci. 70, 3847–3869 (2013). https://doi.org/10.1007/s00018-013-1289-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1289-1