Abstract
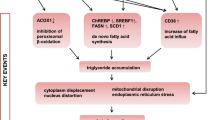
In addition to its well-characterized role in the regulation of drug metabolism and transport by xenobiotics, pregnane X receptor (PXR) critically impacts on lipid homeostasis. In mice, both ligand-dependent activation and knockout of PXR were previously shown to promote hepatic steatosis. To elucidate the respective pathways in human liver, we generated clones of human hepatoma HepG2 cells exhibiting different PXR protein levels, and analyzed effects of PXR activation and knockdown on steatosis and expression of lipogenic genes. Ligand-dependent activation as well as knockdown of PXR resulted in increased steatosis in HepG2 cells. Activation of PXR induced the sterol regulatory element-binding protein (SREBP) 1-dependent lipogenic pathway via PXR-dependent induction of SREBP1a, which was confirmed in primary human hepatocytes. Inhibiting SREBP1 activity by blocking the cleavage-dependent maturation of SREBP1 protein impaired the induction of lipogenic SREBP1 target genes and triglyceride accumulation by PXR activation. On the other hand, PXR knockdown resulted in up-regulation of aldo–keto reductase (AKR) 1B10, which enhanced the acetyl-CoA carboxylase (ACC)-catalyzed reaction step of de novo lipogenesis. In a cohort of human liver samples histologically classified for non-alcoholic fatty liver disease, AKR1B10, SREBP1a and SREBP1 lipogenic target genes proved to be up-regulated in steatohepatitis, while PXR protein was reduced. In summary, our data suggest that activation and knockdown of PXR in human hepatic cells promote de novo lipogenesis and steatosis by induction of the SREBP1 pathway and AKR1B10-mediated increase of ACC activity, respectively, thus providing mechanistic explanations for a putative dual role of PXR in the pathogenesis of steatohepatitis.







Similar content being viewed by others
References
Abdelmalek MF, Diehl AM (2007) Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am 91:1125–1149
Amemiya-Kudo M, Shimano H, Hasty AH et al (2002) Transcriptional activities of nuclear SREBP-1a, −1c, and −2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res 43:1220–1235
Anderson N, Borlak J (2008) Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev 60:311–357
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brunt EM, Janney CG, Di Bisceglie AM et al (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Burk O, Tegude H, Koch I et al (2002) Molecular mechanisms of polymorphic CYP3A7 expression in adult human liver and intestine. J Biol Chem 277:24280–24288
Cheung O, Puri P, Eicken C et al (2008) Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology 48:1810–1820
Cui JY, Gunewardena SS, Rockwell CE, Klaassen CD (2010) ChIPing the cistrome of PXR in mouse liver. Nucleic Acids Res 38:7943–7963
Dai G, He L, Bu P, Wan YJ (2008) Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology 47:1277–1287
Dif N, Euthine V, Gonnet E et al (2006) Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem J 400:179–188
Donnelly KL, Smith CI, Schwarzenberg SJ et al (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115:1343–1351
Farese RV, Walther TC (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860
Fernández-Alvarez A, Tur G, López-Rodas G, Casado M (2008) Reciprocal regulation of the human sterol regulatory element binding protein (SREBP)-1a promoter by Sp1 and EGR-1 transcription factors. FEBS Lett 582:177–184
Geick A, Eichelbaum M, Burk O (2001) Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampicin. J Biol Chem 276:14581–14587
George J, Liddle C (2008) Nonalcoholic fatty liver disease: pathogenesis and potential for nuclear receptors as therapeutic targets. Mol Pharm 5:49–59
Ghose R, Omoluabi O, Gandhi A et al (2011) Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci 89:57–64
Hawkins JL, Robbins MD, Warren LC et al (2008) Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J Pharmacol Exp Ther 326:801–808
He J, Gao J, Xu M et al (2013) PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 62:1876–1887
Hoffart E, Ghebreghiorghis L, Nussler AK et al (2012) Effects of atorvastatin metabolites on induction of drug-metabolizing enzymes and membrane transporters through human pregnane X receptor. Br J Pharmacol 165:1595–1608
Horton JD, Goldstein JL, Brown MS (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109:1125–1131
Joshi A, Rajput S, Wang C et al (2010) Murine aldo–keto reductase family 1 subfamily B: identification of AKR1B8 as an ortholog of human AKR1B10. Biol Chem 391:1371–1378
Khogali AM, Chazan BI, Metcalf VJ, Ramsay JH (1974) Hyperlipidaemia as a complication of rifampicin treatment. Tubercle 55:231–233
Kodama S, Negishi M (2013) PXR cross-talks with internal and external signals in physiological and pathophysiological responses. Drug Metab Rev 45:300–310
Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ (2014) Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146:726–735
Li ZZ, Berk M, McIntyre TM, Feldstein AE (2009) Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 284:5637–5644
Ma J, Yan R, Zu X et al (2008) Aldo–keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J Biol Chem 283:3418–3423
Moreau A, Téruel C, Beylot M et al (2009) A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology 49:2068–2079
Nakamura K, Moore R, Negishi M, Sueyoshi T (2007) Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem 282:9768–9776
Nies AT, Koepsell H, Winter S et al (2009) Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50:1227–1240
Nussler AK, Nussler NC, Merk V et al (2009) The holy grail of hepatocyte culturing and therapeutic use. In: Santin M (ed) Strategies in regenerative medicine. Springer, New York, pp 283–320
Pascussi JM, Robert A, Nguyen M et al (2005) Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest 115:177–186
Reed BD, Charos AE, Szekely AM et al (2008) Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet 4:e1000133
Rysä J, Buler M, Savolainen MJ et al (2013) Pregnane X receptor agonists impair postprandial glucose tolerance. Clin Pharmacol Ther 93:556–563
Sanyal AJ (2005) Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2:46–53
Shimomura I, Shimano H, Horton JD et al (1997) Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 99:838–845
Sookoian S, Castaño GO, Burgueño AL et al (2010) The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet Genomics 20:1–8
Spruiell K, Richardson RM, Cullen JM et al (2014) Role of pregnane X receptor in obesity and glucose homeostasis in male mice. J Biol Chem 289:3244–3261
Starmann J, Fälth M, Spindelböck W et al (2012) Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PLoS One 7:e46584
Sundqvist A, Bengoechea-Alonso MT, Ye X et al (2005) Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 1:379–391
Takagi S, Nakajima M, Mohri T, Yokoi T (2008) Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem 283:9674–9680
Tolson AH, Wang H (2010) Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev 62:1238–1249
Trauner M, Halilbasic E (2011) Nuclear receptors as new perspective for the management of liver diseases. Gastroenterology 140:1120–1125
Tsuzura H, Genda T, Sato S et al (2014) Expression of aldo–keto reductase family 1 member B10 in the early stages of human hepatocarcinogenesis. Int J Mol Sci 15:6556–6568
Wagner M, Zollner G, Trauner M (2011) Nuclear receptors in liver disease. Hepatology 53:1023–1034
Wang C, Yan R, Luo D et al (2009) Aldo–keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. J Biol Chem 284:26742–26748
Zhou J, Zhai Y, Mu Y et al (2006) A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281:15013–15020
Zhou C, Poulton EJ, Grün F et al (2007) The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol 71:220–229
Acknowledgments
We appreciate the expert technical assistance of K. Abuazi-Rincones. The nonprofit foundation Human Tissue and Cell Research (Regensburg, Germany) kindly provided human hepatocytes from four additional donors. This work contains parts of the doctoral thesis of A.B. It was supported, in whole or in part, by the Robert Bosch Foundation, Stuttgart, Germany, by the Interfaculty Center for Pharmacogenomics and Drug Research of the University of Tübingen, Germany, Grant 3-0-0 (to O.B.), by the German Federal Ministry of Education and Research, HepatoSys Grants 0313080I (to O.B.) and 0313081B (to A.K.N.) and Virtual Liver Network Grant 0315755 (to U.M.Z., and M.S.), and by Grant F3008 from the Austrian Science Foundation (to M.T.).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Liver resections of tumor patients, which were used for the preparation of primary human hepatocytes, were performed according to the respective institutional guidelines including the patient’s written informed consent, which were approved by the local ethical committee of the Charité, Humboldt University Berlin, Germany. The human liver biobank study was approved by the local ethical committees of the Charité, Humboldt University, Berlin, and University of Tübingen and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bitter, A., Rümmele, P., Klein, K. et al. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch Toxicol 89, 2089–2103 (2015). https://doi.org/10.1007/s00204-014-1348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1348-x