Abstract
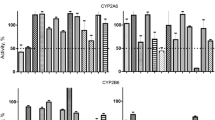
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic heteroatoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic reticulum of hepatocytes and both need oxygen and NADPH as cofactors. The common method to distinguish between the two enzyme systems is to use the thermal inactivation of FMO and to inhibit P450 completely with carbon monoxide, N-octylamine or N-benzylimidazole. In the literature no indication could be found that the heat inactivation of FMO does not affect any of the human P450 enzymes or that the overall P450 inhibitors inhibit the different human P450 enzymes sufficiently and do not affect the FMO. The effect of N-benzylimidazole and heat inactivation was tested on specific activities of seven P450 enzymes in human liver microsomes, 1A2, 2A6, 2C9, 2C19, 2D6, 3A4/5, and 2E1, using methoxyresorufin O-demethylation, coumarin 7-hydroxylation, (S)-warfarin 4-hydroxylation, (S)-(+)-mephenytoin 4-hydroxylation, dextrometorphan O-demethylation, oxidation of denitronifedipine, and chlorzoxazone 6-hydroxylation respectively. The sulfoxidation of methimazole (MMI) was used as a specific probe for the determination of FMO activity. Methimazole sulfoxidation was compared with the well known assay for FMO metabolism, the formation of N,N-dimethylaniline (DMA) N-oxide, to be confirmed as an exclusively FMO mediated reaction. The participation of P450 and FMO in the sulfoxidation of four sulfur containing pesticides, ametryne; terbutryne, prometryne and methiocarb was investigated using human liver microsomes. All four reactions were demonstrated to be catalysed predominantly by cytochrome P450.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 March 1996/Accepted: 20 June 1996
Rights and permissions
About this article
Cite this article
Grothusen, A., Hardt, J., Bräutigam, L. et al. A convenient method to discriminate between cytochrome P450 enzymes and flavin-containing monooxygenases in human liver microsomes. Arch Toxicol 71, 64–71 (1996). https://doi.org/10.1007/s002040050359
Issue Date:
DOI: https://doi.org/10.1007/s002040050359