Abstract
Rationale
Analgesic efficacy of opioids and dosing protocol have been shown to influence analgesic tolerance.
Objective
This study tested the hypothesis that there is an inverse relationship between analgesic efficacy and tolerance following continuous infusion of opioid analgesics. Furthermore, it was hypothesized that analgesic efficacy plays a minor role in determining the magnitude of tolerance following intermittent or acute administration, and that acute and intermittent administration of opioid agonists produces less tolerance than continuous infusion.
Materials and methods
Analgesic (tailflick) efficacy (τ) of etorphine, methadone, oxycodone, and hydrocodone was determined using the operational model of agonism. To induce tolerance, mice were injected with opioid agonists once (acute), once per day for 7 days (intermittent) or continuously infused for 7 days. Dose–response studies were conducted using morphine following treatment.
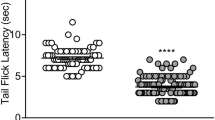
Results
The order of analgesic efficacy was etorphine > methadone > oxycodone ≅ hydrocodone. Infusion of the higher analgesic efficacy drug etorphine produced significantly less tolerance than the lower analgesic efficacy drugs oxycodone, methadone, and hydrocodone at equi-effective doses. In general, intermittent and acute treatment produced less tolerance compared to continuous infusion even at similar daily doses.
Conclusion
Taken together, intermittent and acute opioid agonist administration produces minimal tolerance compared to continuous infusion. Furthermore, there is an inverse relationship between analgesic efficacy and tolerance following continuous infusion. These results suggest that opioid analgesic tolerance may be increased when sustained release dosing formulations or continuous infusions are employed clinically.






Similar content being viewed by others
References
Black JW, Leff P (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162
Chapman CR, Hill HF (1989) Prolonged morphine self-administration and addiction liability. Evaluation of two theories in a bone marrow transplant unit. Cancer 63:1636–1644
Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C (2009) Research gaps on use of opioids for chronic noncancer pain: findings from the review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 10:147–159
Crawford MW, Hickey C, Zaarour C, Howard A, Naser B (2006) Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg 102:1662–1667
Dighe SV, Madia PA, Sirohi S, Yoburn BC (2009) Continuous morphine produces more tolerance than intermittent or acute treatment. Pharmacol Biochem Behav 92:537–542
Duttaroy A, Yoburn BC (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82:1226–1236
Duttaroy A, Kirtman R, Farrell F, Phillips M, Philippe J, Monderson T, Yoburn BC (1997) The effect of cumulative dosing on the analgesic potency of morphine in mice. Pharmacol Biochem Behav 58:67–71
Finney DJ (1973) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, London
Kieffer BL (1999) Opioids: first lessons from knockout mice. Trends Pharmacol Sci 20:19–26
Kumar P, Sunkaraneni S, Sirohi S, Dighe SV, Walker EA, Yoburn BC (2008) Hydromorphone efficacy and treatment protocol impact on tolerance and mu-opioid receptor regulation. Eur J Pharmacol 597:39–45
McCarberg BH (2007) The treatment of breakthrough pain. Pain Med 8:S8–S13
Nicholson B (2009) Benefits of extended-release opioid analgesic formulations in the treatment of chronic pain. Pain Pract 9:71–81
Paronis CA, Holtzman SG (1992) Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther 262:1–9
Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC (2007) Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of µ-opioid receptors and dynamin-2. Eur J Pharmacol 563:92–101
Rosenblum A, Marsch LA, Joseph H, Portenoy RK (2008) Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol 16:405–416
Sirohi S, Dighe SV, Walker EA, Yoburn BC (2008) The analgesic efficacy of fentanyl: relationship to tolerance and μ-opioid receptor regulation. Pharmacol Biochem Behav 91:115–120
Stafford K, Gomes AB, Shen J, Yoburn BC (2001) Mu-opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav 69:233–237
Stevens CW, Yaksh TL (1989) Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J Pharmacol Exp Ther 250:1–8
Walker EA, Young AM (2001) Differential tolerance to antinociceptive effects of µ opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 154:131–142
Wolfram S (1991) A system for doing mathematics by computer. Mathematica. Addison-Wesley, Reading
Yoburn BC, Chen J, Huang T, Inturrisi CE (1985a) Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther 235:282–286
Yoburn BC, Cohen A, Umans JG, Ling GS, Inturrisi CE (1985b) The graded and quantal nature of opioid analgesia in the rat tailflick assay. Brain Res 331:327–336
Zernig G, Issaevitch T, Broadbear JH, Burke TF, Lewis JW, Brine GA, Woods JH (1995) Receptor reserve and affinity of mu opioid agonists in mouse antinociception: correlation with receptor binding. Life Sci 57:2113–2125
Zernig G, Issaevitch T, Woods JH (1996) Calculation of agonist efficacy, apparent affinity, and receptor population changes after administration of insurmountable antagonists: comparison of different analytical approaches. J Pharmacol Toxicol Methods 35:223–237
Acknowledgements
The authors thank Mohit Pawar and Shakira Weir for technical assistance. Dr. M.T. Turnock assisted the authors with concept development and frequent discussions during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
These studies were supported in part by DA 19959 to BCY.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 31 kb)
Rights and permissions
About this article
Cite this article
Madia, P.A., Dighe, S.V., Sirohi, S. et al. Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology 207, 413–422 (2009). https://doi.org/10.1007/s00213-009-1673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1673-6