Abstract
The transient receptor potential channel TRPV5 is localized to the apical membrane of the distal renal tubule and plays an important role in the regulation of transepithelial Ca2+ reabsorption in kidney. We have previously reported that extracellular protons inhibit TRPV5 by binding to glutamate-522 (E522) in the extracellular domain of the channel. We suggested that E522 is an extracellular “pH sensor” and its titration by extracellular protons inhibits TRPV5 via conformational change(s) of the pore helix. We now report that mutation of a pore helix residue glutamate-535 to glutamine (E535Q) enhances the sensitivity of the channel to inhibition by extracellular protons (i.e., shifting the apparent pKa for inhibition by extracellular protons to the more alkaline extracellular pH). The enhancement of extracellular proton-mediated inhibition of E535Q mutant is also dependent on E522. We have also reported that intracellular acidification enhances the sensitivity of TRPV5 to inhibition by extracellular protons. We now find that modulation of the extracellular proton-mediated inhibition by intracellular acidification is preserved in the E535Q mutant. These results provide further support for the idea that pore helix is involved in the regulation of TRPV5 by extracellular protons. Inhibition of TRPV5 by extracellular protons may contribute to hypercalciuria in diseases associated with high acid load.
Similar content being viewed by others
Introduction
The kidney is critical for maintaining calcium homeostasis. To maintain calcium balance, ∼98% of Ca2+ load filtered by the glomerulus must be reabsorbed along the nephron (Suki, Lederer & Rouse, 2000). Approximately 78% of the filtered load of Ca2+ is reabsorbed in the proximal tubule and the thick ascending limb (TAL) of Henle’s through the paracellular pathway. The remaining ∼20% of Ca2+ reabsorption in kidney occurs via a transcellular pathway in the distal part of the nephron, consisting of distal convoluted tubules, connecting tubules and the initial portion of the cortical collecting ducts. The transcellular reabsorption of Ca2+ in the distal nephron involves multiple steps. The first step is passive entry of Ca2+ through the Ca2+ channels in the apical membranes, followed by facilitated diffusion of Ca2+ through cytosol via the 1,25- dihydroxyvitamin-D3 (1,25-D3)-dependent Ca2+-binding protein calbindin-D28K (Sooy, Kohut & Christakos, 2000) and eventually extrusion of Ca2+ across the basolateral membranes via Na+/Ca2+ exchangers and/or Ca2+-ATPases. The initial step of passive entry through Ca2+ channels in the apical membranes likely constitutes the rate-limiting step of the transepithelial Ca2+ reabsorption in the distal nephron (Suki et al., 2000). It is a major target for regulation by hormones that control Ca2+ homeostasis. These hormones include parathyroid hormone, 1,25-D3, calcitonin, prostaglandin E2 and arginine vasopressin (Hoenderop, Nilius & Bindels, 2002).
The cDNAs for the apical Ca2+ channels have recently been isolated. Hoenderop et al. (1999) and Peng et al. (1999) isolated cDNA from rabbit kidney and rat intestine and named it ECaC1 (for epithelial Ca2+ channel) and CaT1 (for Ca2+ transporter protein), respectively. The epithelial Ca2+ channels ECaC1 and CaT1 belong to the superfamily of transient receptor potential (TRP) channels, which include the TRPC, TRPV, TRPM TRPP, TRPML, TRPN and TRPA subfamilies (Clapham, 2003; Huang, 2004). The TRPV subfamily – named after its first mammalian member, vanilloid receptor 1 (VR1) – contains six mammalian members, TRPV1-6 (Jordt, McKemy & Julius, 2003). ECaC1 and CaT1 are members 5 and 6 of the V-type TRP channels (TRPV5 and TRPV6), respectively (Montell et al., 2002). TRPV5 and TRPV6 are the only two highly Ca2+-selective TRP channels and mediate transepithelial Ca2+ transport in kidney and intestine (Hoenderop et al., 2002; Huang, 2004). The transmembrane topology of TRP channels is similar to that of voltage-gated Ca2+ and K+ channels and cyclic nucleotide-gated (CNG) cation channels in having an amino-terminal cytoplasmic region, six membrane-spanning domains with an intervening putative pore-forming region similar to other Ca2+-permeable channels and a carboxyl-terminal cytoplasmic terminus (Clapham, 2003; Hoenderop et al., 2002; Huang, 2004).
Our understanding of the structure of ion channels has been markedly advanced by recent studies on the three-dimensional structure of the bacterial K+ channel KcsA and related channels. The KcsA channel consists of four identical subunits (Doyle et al., 1998). Each subunit contains two membrane-spanning helices (organized as inner and outer helices) and an intervening pore-forming (P-) loop, consisting of the glycine-tyrosine-glycine-threonine-valine (GYGTV) signature sequence for all K+ channels and a preceding pore helix region. The carbonyl oxygen atoms of amino acids of the signature sequence form the selectivity filter, which is stabilized by pore helices. The four inner helices pack against each other as a bundle near the intracellular aspect of the membrane, giving an appearance of an inverted teepee. This structure of KcsA suggests two potential activation gates, an outer selectivity filter gate stabilized by pore helices and an inner “bundle-crossing” gate. This architecture is likely a general structure for all P-loop-containing channels, including voltage-gated K+ channels, inward rectifier and Ca2+-activated K+ channels and CNG channels (Flynn, Johnson & Zagotta, 2001). The three-dimensional structure of TRP channels is not known. However, several recent studies have provided information on the pore region of TRPV5 and the highly homologous TRPV6. Mutation of aspartate-542 of TRPV5 greatly reduces Ca2+ permeation, indicating that aspartate-542 lies within the selectivity filter (Nilius et al., 2001). A region of ∼15 amino acids in the preselectivity filter region of TRPV5 and TRPV6 is highly homologous to that in KcsA and likely forms the pore helix of TRPV5 and TRPV6 (Dodier et al., 2004; Voets et al., 2004, Yeh et al., 2005).
TRPV5 is localized to the apical membrane of polarized epithelia and critically controls transepithelial Ca2+ transport (Hoenderop et al., 2002). Reduction of transepithelial Ca2+ transport is observed in many conditions associated with overproduction of acids (Breslau et al., 1988; Sutton, Wong & Dirks, 1979). We have recently reported that both extracellular and intracellular protons reduce open probability of TRPV5 to inhibit the channel (Yeh et al., 2003, 2005). The extracellular and intracellular protons inhibit TRPV5, at least in part, by binding to glutamate-522 and lysine-607 in the extracellular and intracellular domains of the channel, respectively (Yeh et al., 2003, 2005). Intracellular acidification enhances inhibition of TRPV5 by extracellular protons and vice versa. We have also shown that intracellular acidification causes a conformational change of the pore helix consistent with a clockwise rotation along its long axis and suggested that the conformational change is important for cross-regulation of the channel by intracellular and extracellular protons. To support the role of pore helix in the regulation by extracellular protons, we now report that mutation of a pore helix residue glutamate-535 to glutamine (E535Q) enhances the sensitivity of the channel to inhibition by extracellular protons and that the enhancement of extracellular proton-mediated inhibition of the E535Q mutant is abolished by mutation of E522. These results support the idea that E522 of TRPV5 is an extracellular pH sensor and that titration of E522 by extracellular protons inhibits the channel via conformational changes of the pore helix.
Materials and Methods
MOLECULAR BIOLOGY AND CELL CULTURE
Mutant rabbit TRPV5s in a pCDNA3 mammalian expression vector were constructed using a commercial site-directed mutagenesis kit (QuikChange kit; Stratagene, La Jolla, CA) as previously described (Yeh et al., 2003, 2005). Chinese hamster ovary (CHO) cells (at ∼50% confluence) were cotransfected with cDNA for enhanced green fluorescent protein (pEGFP) (0.5 μg) plus cDNAs for wild-type (WT) or mutant TRPV5 (0.5–5 μg) using lipofectamine-plus transfection kits (GIBCO, Grand Island, NY) as described. About 24–48 h after transfection, cells were dissociated by brief exposure to trypsin for ∼30 s, followed by repetitive pipetting and placement in a chamber for recording. Transfected cells were identified using epifluorescent microscopy for recordings. In some experiments, cells attached to cover glass (without trypsin treatment) were used for recording. TRPV5 expression and regulation by pH were not different between cells treated and untreated with trypsin.
ELECTROPHYSIOLOGICAL RECORDINGS
Whole-cell patch-clamp recordings were performed using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Burlingame, CA) (Yeh et al., 2003, 2005). For whole-cell recordings, the pipette and bath solution contained (in mM) 140 Na-Asp (sodium aspartate), 6 CsCl, 1 ethylenediaminetetraacetic acid (EDTA), 10 glucose, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, titrated to pH as specified) and 120 Na-Asp, 20 CsCl, 10 EDTA, 10 HEPES (pH as specified), respectively. Mg2-free solutions were used as extracellular and intracellular Mg2+ inhibits TRPV5 (Nilius et al., 2001; Lee at al., 2005). Holding potential was 0 mV. The voltage stimulus consisted of a 50-ms hyperpolarization step (−100 mV) and ramp from −100 to + 100 mV over 400 ms. Currents were low-pass filtered at 1 kHz using an eight-pole Bessel filter, sampled every 0.1 ms (10 kHz) with a Digidata−1300 interface and stored directly onto a computer hard disk using pCLAMP9 software (Axon Instruments, Foster City, CA). Data were transferred to compact disks for long-term storage. To test the accessibility of substituted cysteines, MTSET (Toronto Research Chemicals, Toronto, Canada) was added to the bath solution from 100 mM stocks. To determine the relative permeability of K+, Li+ and Cs+ to Na+ in whole-cell recordings, the initial bath and pipette solution contained (in mM) 130 Na-Asp, 10 NaCl, 1 EDTA, 10 HEPES (pH as specified) and 130 Na-Asp, 10 NaCl, 10 EDTA, 10 HEPES (pH as specified), respectively. Na-Asp-containing bath solution was then replaced by one that contains 130 X-Asp, 10 X-Cl, 10 HEPES, 1 EDTA (X is K, Li or Cs).
For cell-attached single-channel recording (Yeh et al., 2003), the pipette and bath solution contained 140 Na-Asp, 10 NaCl, 1 EDTA, HEPES 10 (pH as specified) and 140 K-Asp, 10 NaCl, 1 EDTA, 10 HEPES (pH 7.4), respectively. Currents were low-pass filtered at 1 kHz using an eight-pole Bessel filter, sampled every 0.1 ms (10 kHz) with a Digidata−1300 interface and stored directly onto computer hard disk using pCLAMP9 software. Data were transferred to compact disk for long-term storage. Single-channel current amplitude and open probability were analyzed using the Clampfit9 program of pCLAMP9 software (Yeh et al., 2003). Open probability was analyzed from recordings that showed one active channel over >5 min.
DATA ANALYSIS
To analyze the channel sensitivity to inhibition by extracellular or intracellular protons, relative currents at different pH values were fitted with a modified Hill equation using the Sigma-Plot program (SPSS Inc., Chicago, IL) (Yeh et al., 2003, 2005). Inward currents at −100 mV were analyzed. Lanthanum-insensitive residual current (in 1 mM La3+) was subtracted. Relative current was normalized to the maximal current (maximal current is labeled “1”). The permeability ratios of different monovalent cations vs. Na+ were calculated based on the shifts of reversal potential (ΔE rev) on exchanging the extracellular solutions, P X/P Na = exp(ΔE rev x F/RT), where R, T and F have the usual meanings (Hille, 2001). Data are shown as mean ± standard error of the mean (SEM) of the number of observations. Statistical comparison was made using unpaired Student’s t-test.
Results
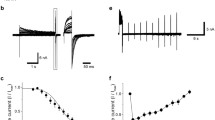
Amino acids 527–538 in the preselectivity filter region of TRPV5 form the pore helix (Dodier et al., 2004; Voets et al., 2004; Yeh et al., 2005) (Fig. 1A). We have previously shown that extracellular protons inhibit TRPV5, at least in part, by binding to glutamate−522 (E522) in the extracellular domain (Yeh et al., 2003). We proposed that binding of protons to the extracellular “pH sensor” causes narrowing of the selectivity filter, likely via conformational change(s) of the pore helix (e.g., rotation shown in Fig. 1A). To further examine the role of the pore helix in this process, we mutated every amino acid in the pore helix individually and tested the effects on the regulation by extracellular protons. CHO cells expressing each mutant were recording by whole-cell patch-clamp recording at various extracellular pHs (Fig. 1B). Currents in response to the voltage ramp stimuli (−100 to +100 mV) were measured. Figure 1C shows representative whole-cell currents through the glutamate−535 to glutamine (E535Q) mutant at different extracellular pHs. Extracellular acidification from pH 9.4 to 5.4 caused reduction of currents. The effects of extracellular pH were reversible (not shown). To analyze the effect of extracellular protons on the channel, the relative magnitude of current was plotted against extracellular pH (pHe). As shown in Figure 1D, the E535Q mutant exhibited increased sensitivity to the extracellular protons compared to the WT channel. The apparent pKa for pHe regulation of the E535Q mutant and WT channels were 7.75 ± 0.32 (n = 5) and 6.44 ± 0.10 (n = 32), respectively (p < 0.05). Mutations of other amino acids of the pore helix did not alter the sensitivity for pHe regulation (i.e., pKa for pHe regulation for each mutant is not different from that of WT channel; not shown). Glutamate is a titratable amino acid. However, if E535 functions as a pH sensor, mutation of the residue to glutamine would decrease the sensitivity of the channel to protons. The increase (instead of decrease) in sensitivity to extracellular protons for the E535Q mutant indicates that E535 does not function as a pH sensor.
Effect of E535Q mutation on pHe regulation of TRPV5. (A) Transmembrane segment 5 (TM5) and TM6 and pore region (pore helix and selectivity filter) of TRPV5. TRPV5 is tetrameric. Only one subunit is shown. E522 is a pHe sensor. K607 is a pHi sensor. E535 is located at the distal end of the pore helix. (B) Whole-cell configuration and voltage-ramp protocol for recording. pHe was varied from 4.4 to 9.4. pHi was 7.2. Holding potential was 0 mV. Voltage stimulus consisted of a 50-ms hyperpolarization step and ramp from −100 to +100 mV over 400 ms. Scale bar for 100 ms is shown. (C) Representative current tracing at different pHe values in response to the voltage ramp. Dotted horizontal line indicates current level of 0 nA. Membrane voltage at −100, 0 and +100 mV indicated by arrows. Scale bars for current (1 nA) and time (100 ms) are shown. (D) Dose-response curve for inhibition of E535Q and WT TRPV5 by extracellular protons. Inward current at −100 mV was used for analysis. Lanthanum-insensitive residual current (10 mM La3+) was subtracted. Relative current (normalized to maximal current, maximal current labeled 1) at any given pHe is shown. Current density (pA/pF) values for E535Q mutant and WT TRPV5 (at pHe 9.4) were 1,120 ± 156 (n = 5) and 1,250 ± 59 (n = 32), respectively, indicating that E535Q mutation did not affect expression of the channel. (E, F) Relative permeability of inorganic monovalent cations to Na+ for E535Q mutant. Whole-cell currents were first recorded in a bath solution containing 130 mM Na-Asp, which was subsequently replaced by solutions containing 130 mM X-Asp (X is Li, Cs or K). Shown are I-V curves at these different extracellular cations. (E) I-V curves in the full range. (F) I-V curves of the boxed regions (indicated in E) in an expanded scale.
To see if the E535Q mutation affects the selectivity filter, we measured the relative selectivity to inorganic monovalent cations. Permeability ratios of Li+, K+ and Cs+ relative to Na+ were determined by shifts of reversal potentials when extracellular Na+ was replaced by respective ions (Fig. 1E, F). The relative permeability ratios of Li+, K+ and Cs+ to Na+ were calculated at 0.89 ± 0.04, 0.78 ± 0.04 and 0.66 ± 0.05, respectively (see Materials and Methods for equation). This selectivity sequence, Na+ > Li+ > K+ > Cs+, is identical to the WT channel (Yeh et al., 2005), suggesting that the E535Q mutation does not alter the basic structure of the selectivity filter.
E522 is an extracellular pH sensor (Yeh et al., 2003). To see if the sensitivity of the E535Q mutant to extracellular protons is mediated by pH sensing via E522, we examined the effect of extracellular protons on the E535Q/E522Q double mutant (Fig. 2A). As shown in Figure 2B, mutation of E522 decreased the increase in sensitivity to extracellular protons caused by E535Q mutation. The pKa for pHe regulation for the E535Q/E522Q double mutant was 6.37 ± 0.3 (n = 4) (p < 0.05 vs. 7.75 ± 0.32 for E535Q). Sensing by E522 is not the only mechanism for regulation of TRPV5 by extracellular pH. The E522Q mutant remains partially sensitive to extracellular protons (Yeh et al., 2003). We found that this is due to the fact that extracellular acidification decreases both open probability and single-channel conductance of TRPV5 (Yeh et al., 2003). E522Q mutation prevents the extracellular acidification-induced decrease of open probability (but not single-channel conductance) of TRPV5.
Effect of E522Q mutation on pHe regulation of E535Q mutant. (A) Representative current tracing at different pHe values for the E535Q/E522Q double mutant. Dotted horizontal line indicates current level of 0 nA. Membrane voltage at −100, 0 and +100 mV indicated by arrows. Scale bars for current (1 nA) and time (100 ms) are shown. (B) Dose-response curve for inhibition of WT, E535Q and E535Q/E522Q double mutant by extracellular protons. Inward current at −100 mV was used. Lanthanum-insensitive residual current (10 mM La3+) was subtracted. Relative current (normalized to maximal current, maximal current labeled 1) at any given pHe is shown. The results for WT and E535Q mutant are from Figure 1 and reshown here for comparison with E535Q/E522Q. (C) Representative cell-attached single-channel recordings (at −100 mV) of E535Q and E535Q/E522Q mutants at pHe 7.0. Labels C and O in panel C indicate closed and open states, respectively. Scale bars for current amplitude and time are shown. (D) Single-channel open probability for WT and mutants (n = 4–6 for each).
Like the E522Q mutant, E535Q/E522Q remained partially sensitive to extracellular protons (Fig. 2B). We next examined single-channel properties of WT TRPV5, E522Q, E535Q and E535Q/E522Q by cell-attached recordings at pHe 7.0 (Fig. 2C). As shown in Figure 2C, single-channel conductance of E535Q and E535Q/E522Q were 85 ± 6.2 and 87 ± 4.6 pS, respectively. These values are not different from that of WT TRPV5 and the E522Q mutant at pHe 7.0 (not shown; see Yeh et al., 2003). These results indicate that changes in pHe sensitivity by E535Q and/or E522Q mutation are not through changes of single-channel conductance but rather through changes of open probability. In support of this interpretation, we found that open probability (Po) values for WT TRPV5, E522Q, E535Q and E535Q/522Q were 0.65 ± 0.08, 0.88 ± 0.12, 0.45 ± 0.06 and 0.85 ± 0.11, respectively (Fig. 2D). The lower Po for E535Q relative to WT (0.45 ± 0.06 vs. 0.65 ± 0.08, p < 0.05) indicates that a decrease in Po underlies the increased sensitivity to extracellular protons by E535Q mutation. The similarity in Po between E522Q and E535Q/E522 (0.88 ± 0.12 vs. 0.85 ± 0.11, not significant) indicates that sensing of extracellular protons by E522 mediates the decrease in Po caused by E535Q mutation.
We have shown that intracellular acidification enhances the sensitivity of TRPV5 to inhibition by extracellular protons (Yeh et al., 2005). We next examined if pHe regulation of E535Q mutant remains sensitive to modulation by intracellular protons. In the experiments shown in Figures 1 and 2, the effects of varying pHe on TRPV5 channel activity were examined at intracellular pH 7.2. In the following experiments, effects of varying pHe on channel activity were examined at different intracellular pH (pHi): 6.8, 7.2 and 8.4. As shown in Figure 3A, decreasing pHi from 7.2 to 6.8 shifted the sensitivity of E535Q mutant to extracellular protons to more alkaline pHe, indicating increased sensitivity to extracellular protons. Conversely, increasing pHi from 7.2 to 8.4 decreased the sensitivity to extracellular protons. The average pKa values for pHe regulation of E535Q mutant at pHi 8.4, 7.2 and 6.8 were 6.67 ± 0.22 (n = 4), 7.75 ± 0.32 (n = 5) and 8.24 ± 0.34 (n = 4), respectively (Fig. 3B).
Effects of pHi on pHe regulation of E535Q mutant. (A) Dose-response curve for inhibition of E535Q mutant by extracellular protons at pHi 6.8, 7.2 or 8.4. Inward current at −100 mV was used for analysis. Lanthanum-insensitive residual current (10 mM La3+) was subtracted. Relative current (normalized to maximal current, maximal current labeled 1) at any given pHe is shown. (B) Apparent pKa for pHe regulation of E535Q mutant at pHi 6.8, 7.2 or 8.4.
We also found that TRPV5 is inhibited by intracellular protons (Yeh et al., 2005). Lysine−607 (K607) is an intracellular pH sensor (see Fig. 1A); mutation of the residue to asparagine (K607N) decreases sensitivity of the channel to inhibition by intracellular protons (Yeh et al., 2005). To see if modulation of pHe regulation of E535Q by intracellular protons is mediated by pH sensing via K607, we examined the effects of varying pHe on the E535Q/K607N double mutant at different pHi values: 6.8, 7.2 and 8.4 (Fig. 4A). As shown in Figure 4A, the titration curves for pHe regulation of the E535Q/K607N double mutant at pHi 6.8, 7.2 and 8.4 were similar. The average pKa values for pHe regulation (mean ± SEM) of the E535Q/K607N double mutant at pHi 8.4, 7.2 and 6.8 were 6.07 ± 0.27 (n = 4), 6.31 ± 0.30 (n = 5) and 6.41 ± 0.34 (n = 4), respectively (Fig. 4B). These results indicate that the modulation by intracellular protons of pHe regulation of the E535Q mutant is mediated by K607 (see Fig. 3B for comparison).
Role of K607 in the modulation by intracellular protons of pHe regulation of E535Q. (A) Dose-response curve for inhibition of E535Q/K607N double mutant by extracellular protons at pHi 6.8, 7.2 or 8.4. Inward current at −100 mV was used. Lanthanum-insensitive residual current (10 mM La3+) was subtracted. Relative current (normalized to maximal current, maximal current labeled 1) at any given pHe is shown. (B) Apparent pKa for pHe regulation of E535Q/K607N double mutant at pHi 6.8, 7.2 or 8.4 (closed circles). The results for E535Q mutant are from Figure 3 and reshown here (open circles) for comparison.
We next used the substituted cysteine accessibility method to investigate if mutation of E535 affects the structure of pore helix and the rotation mechanism. The membrane-impermeant, sulfhydryl-reacting reagent MTSET modifies cysteine-substituted amino acids and causes inhibition of ion currents. By using the inhibition of currents by extracellular methanethiosulfonate ethyltrimethylammonium (MTSET), it has been shown that amino acids of the pore helix of TRPV5 are packed in a right-handed α-helical pattern and separated into water-accessible (MTSET-reactive) and water-inaccessible (MTSET-nonreactive) surfaces (Dodier et al., 2004; Voets et al., 2004, Yeh et al., 2005). We have further shown that intracellular acidification alters the reactivity of TRPV5 to MTSET in a pattern consistent with a clockwise rotation along the long axis (see Fig. 6 of Yeh et al., 2005). Here, we examined the sensitivity of three representative cysteine-substituted amino acids of the pore helix of E535Q (alanine−529, leucine−530 and phenylalanine−531) to extracellular MTSET (Fig. 5A). We found that the E353Q/A529C mutant is sensitive to MTSET at both pHi 8.4 and 6.8, E535Q/L530C is sensitive at pHi 8.4 but not at 6.8 and E535Q/F531C mutant is insensitive at both pHi 8.4 and 6.8 (Fig. 5B-D, respectively). This pattern is essentially the same as that for cysteine-substituted WT TRPV5 (Yeh et al., 2005).
Discussion
The structure and mechanism involved in the gating of TRP channels are largely unknown. Based on the homology with KcsA and functional studies using the substituted cysteine accessibility method (Doyle et al., 1998; Dodier et al., 2004; Voets et al., 2004, Yeh et al., 2005), it is believed that amino acids 527–539 of TRPV5 amino-terminal to the selectivity filter form the pore helix. To understand the gating of TRPV5 by protons, we have previously shown that both extracellular and intracellular protons inhibit TRPV5 (Yeh et al., 2003, 2005). The effects of extracellular and intracellular protons occur, at least partly, via binding to E522 and K607 in the extracellular and intracellular domains, respectively (Yeh et al., 2003, 2005). We have also shown that the regulation by extracellular protons and by intracellular protons cross talk and that intracellular acidification causes a conformational change of the pore helix consistent with a clockwise rotation along its long axis and leads to narrowing of the selectivity filter (Yeh et al., 2005). As the effects of extracellular and intracellular protons on the selectivity filter are similar, we proposed that binding of extracellular protons to E522 causes a similar conformational change in the pore helix (Yeh et al., 2005). The pore helix of ion channels is important for ion permeation. In the crystal structure of KcsA, amino acids of the pore helices interact with and stabilize the selectivity filter (Doyle et al., 1998). The pore helices of KcsA are oriented such that the partially negative charge of the oriented pore helices points toward a water-filled cavity at the membrane center, providing a favorable electrostatic force for stabilization of K+ ions. We suggested that conformational change(s) of the pore helix of TRPV5 caused by intracellular acidification causes misalignments of the helix dipoles to close the channel.
However, in the previous study, we could not perform experiments using the substituted cysteine accessibility method to show that extracellular acidification causes conformational changes of the pore helix. This was due to the fact that the intracellular domain of TRPV5 contains at least 14 cysteine residues, preventing application of cysteine-modifying reagents from inside. Mutation of some of the intracellular cysteine residues renders the channel nonfunctional (not shown). Also, the reactivity of cysteine-modifying reagents is pH-dependent, preventing examination of the effects of pHe on the pore helix by extracellular application of the reagents. In the present study, we have made mutations of amino acids in the pore helix and found that mutation of pore helix residue E535 alters pHe sensitivity. These results provide further support for the idea that the pore helix is involved in the regulation of TRPV5 by extracellular protons.
How does mutation of E535 in the pore helix increase the sensitivity of TRPV5 to extracellular protons? The mechanism of gating of TRPV5 by extracellular protons likely involves a series of steps, including sensing of protons by E522, the mechanics of opening and closing the gate (i.e., rotation of pore helix leading to narrowing of the selectivity filter) and in-between step(s) of chemicomechanical transduction that relay the pH sensing to the mechanics of gating. In the present study, we found that E522Q mutation abolishes the increased sensitivity of E535Q to extracellular protons (Fig. 2), indicating that the E535Q mutant can sense extracellular protons via E522. Thus, E535Q mutation does not alter the pH-sensing step. We also found that the modulation by intracellular protons of pHe regulation of E535Q mutation (Fig. 3) is similar to that of the WT channel (see Fig. 3B of Yeh et al., 2005), suggesting that the basic mechanics of gating via rotation of the pore helix are intact. This idea is further supported by the finding that intracellular acidification causes rotation of the pore helix of E535Q (Fig. 5) similar to that for WT channel (see Fig. 6 of Yeh et al., 2005). In addition, E535Q mutation does not affect the selectivity filter (Fig. 1E, F). Thus, E535Q mutation most likely increases the sensitivity of TRPV5 to extracellular protons by altering the chemicomechanical transduction step. The chemicomechanical transduction may include conformational changes of amino acids between E522 and the pore helix and coupling of the conformational changes to rotation of the pore helix. E535Q mutation may alter the conformational changes and/or the coupling efficiency.
E535 is the only acidic residue in the pore helix of TRPV5 (Dodier et al., 2004; Yeh et al., 2005). The acidic residue equivalent to E535 is conserved in the pore helix of KcsA and inwardly rectifying K+ channels but not in other channels, including the Shaker K+ channel and CNG channels (Doyle et al., 1998). How E535 of TRPV5 participates in relaying proton sensing by E522 to conformational changes of the pore helix awaits future studies.
TRPV5 is the gatekeeper of transepithelial Ca2+ reabsorption in the kidney (Hoenderop et al., 2002). Extracellular acidosis inhibits renal Ca2+ reabsorption, causing hypercalciuria (Breslau et al., 1988; Huang, 2004; Sutton et al., 1979). Hypercalciuria predisposes to kidney stone formation (Breslau et al., 1988). One mechanism for hypercalciuria during chronic metabolic acidosis is downregulation of TRPV5 (Nijenhuis et al., 2006). Direct inhibition of TRPV5 by protons may also contribute to hypercalciuria and kidney stone formation in diseases associated with high acid load.
References
Breslau N.A., Brinkley L., Hill K.D., Pak C.Y.C. 1988. Relationship between animal protein-rich diet to kidney stone formation and calcium metabolism. J. Clin. Endocrinol. Metab. 66:140–146
Clapham D.E. 2003. TRP channels as cellular sensors. Nature 426:517–524
Dodier Y., Banderali U., Klein H., Topalak O., Dafi O., Simoes M., Bernatchez G., Sauve R., Parent L. 2004. Outer pore topology of the ECaC-TRPV5 channel by cysteine scan mutagenesis. J. Biol. Chem. 279:6853–6862
Doyle D.A., Morais-Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280:69–77
Flynn G.E., Johnson J.P. Jr., Zagotta W.N. 2001. Cyclic nucleotide-gated channels: Shedding light on the opening of a channel pore. Nat. Rev. Neurosci. 2:643–651
Hille B. 2001. Ion Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA
Hoenderop J.G., van der Kemp A.W., Hartog A., van de Graaf S.F., van Os C.H., Willems P.H., Bindels R.J. 1999. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 274:8375–8378
Hoenderop J.G., Nilius B., Bindels R.J. 2002. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu. Rev. Physiol. 64:529–549
Huang C.-L. 2004. The transient receptor potential superfamily of ion channels. J. Am. Soc. Nephrol. 15:1690–1699
Jordt S.E., McKemy D.D., Julius D. 2003. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr. Opin. Neurobiol. 13:487–492
Lee J., Cha S.-K., Sun T.-J., Huang C.-L. 2005. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J. Gen. Physiol. 126:439–451
Montell C., Birnbaumer L., Flockerzi V., Bindels R.J., Bruford E.A., Caterina M.J., Clapham D.E., Harteneck C., Heller S., Julius D., Kojima I., Mori Y., Penner R., Prawitt D., Scharenberg A.M., Schultz G., Shimizu N., Zhu M.X. 2002. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 92:229–231
Nijenhuis T., Renkema K.Y., Hoenderop J.G., Bindels R.J. 2006. Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J. Am. Soc. Nephrol. 17:617–626
Nilius B., Vennekens R., Prenen J., Hoenderop J.G., Droogmans G., Bindels R.J. 2001. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J. Biol. Chem. 276:1020–1025
Peng J.B., Chen X.Z., Berger U.V., Vassilev P.M., Tsukaguchi H., Brown E.M., Hediger M.A. 1999. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 274:22739–22746
Sooy K., Kohut J., Christakos S. 2000. The role of calbindin and 1,25-dihydroxyvitamin D3 in the kidney. Curr. Opin. Nephrol. Hypertens. 9:341–347
Suki W.N., Lederer E.D., Rouse D. 2000. Renal transport of calcium, magnesium, and phosphate. In: Brenner B.M. editor. Brenner & Rector’s The Kidney, 6th ed., Saunders, Philadelphia pp. 520–574
Sutton R.A., Wong N.L., Dirks J.H. 1979. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int. 15:520–533
Voets T., Janssens A., Droogmans G., Nilius B. 2004. Outer pore architecture of a Ca2+-selective TRP channel. J. Biol. Chem. 279:15223–15230
Yeh B.-I., Sun T.-J., Lee J.Z., Chen H.-H., Huang C.-L. 2003. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J. Biol. Chem. 278:51044–51052
Yeh B.-I., Kim Y.K., Jabbar W., Huang C.-L. 2005. Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J. 24:3224–3234
Acknowledgement
We thank Dr. In Deok Kong for technical assistance and critical reading of the manuscript. This study was supported by Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A06-0043418) (to B.-I. Y.) and by National Institutes of Health grant DK−20543 (to C.-L. H.). C.-L. H. holds the Jacob Lemann Professorship in Calcium Transport of the University of Texas Southwestern Medical Center.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yeh, BI., Yoon, J. & Huang, CL. On the Role of Pore Helix in Regulation of TRPV5 by Extracellular Protons. J Membrane Biol 212, 191–198 (2006). https://doi.org/10.1007/s00232-006-0023-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0023-4