Abstract
Purpose
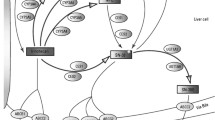
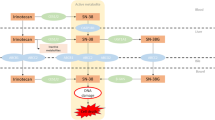
Effects of genetic polymorphisms/variations of ABCB1, ABCC2, ABCG2 and SLCO1B1 in addition to “UGT1A1*28 or *6” on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients were investigated.
Methods
Associations between transporter haplotypes/variations along with UGT1A1*28 or *6 and SN-38 area under the time–concentration curve (AUC) or neutropenia were examined in irinotecan monotherapy (55 patients) and irinotecan–cisplatin-combination therapy (62 patients).
Results
Higher SN-38 AUC values were observed in ABCB1 2677G>T (A893S) (*2 group) for both regimens. Associations of grade 3/4 neutropenia were observed with ABCC2 −1774delG (*1A), ABCG2 421C>A (Q141K) and IVS12 + 49G>T (# IIB) and SLCO1B1 521T>C (V174A) (*15 · 17) in the irinotecan monotherapy, while they were evident only in homozygotes of ABCB1*2, ABCG2 # IIB, SLCO1B1*15 · 17 in the cisplatin-combination therapy. With combinations of haplotypes/variations of two or more genes, neutropenia incidence increased, but their prediction power for grade 3/4 neutropenia is still unsatisfactory.
Conclusions
Certain transporter genotypes additively increased irinotecan-induced neutropenia, but their clinical importance should be further elucidated.



Similar content being viewed by others
References
Slatter JG, Su P, Sams JP, Schaaf LJ, Wienkers LC (1997) Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos 25:1157–1164
Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ (1998) Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 15:847–854
Ciotti M, Basu N, Brangi M, Owens IS (1999) Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38) by the human UDP-glucuronosyltransferases encoded at the UGT1 locus. Biochem Biophys Res Commun 260:199–202
Gagne JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C (2002) Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). Mol Pharmacol 62:608–617
Haaz MC, Rivory L, Riché C, Vernillet L, Robert J (1998) Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res 58:468–472
Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD (2003) Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat 6:71–84
Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I (2005) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33:434–439
Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60:6921–6926
Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2:43–47
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388
Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Jang IJ, Lee DH, Lee JS (2006) Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 24:2237–2244
Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, Kaniwa N, Sawada J, Hamaguchi T, Yamamoto N, Shirao K, Yamada Y, Ohmatsu H, Kubota K, Yoshida T, Ohtsu A, Saijo N (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: Roles of UGT1A1*6 and *28. Pharmacogenet Genomics 17:497–504
Jada SR, Lim R, Wong CI, Shu X, Lee SC, Zhou Q, Goh BC, Chowbay B (2007) Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patients. Cancer Sci 98:1461–1467
Sai K, Saito Y, Sakamoto H, Shirao K, Kurose K, Saeki M, Ozawa S, Kaniwa N, Hirohashi S, Saijo N, Sawada J, Yoshida T (2008) Importance of UDP-glucuronosyltransferase 1A1*6 for irinotecan toxicities in Japanese cancer patients. Cancer Lett 261:165–171
Mathijssen RH, Marsh S, Karlsson MO, Xie R, Baker SD, Verweij J, Sparreboom A, McLeod HL (2003) Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin Cancer Res 9:3246–3253
Sai K, Kaniwa N, Itoda M, Saito Y, Hasegawa R, Komamura K, Ueno K, Kamakura S, Kitakaze M, Shirao K, Minami H, Ohtsu A, Yoshida T, Saijo N, Kitamura Y, Kamatani N, Ozawa S, Sawada J (2003) Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype-dependent renal clearance of irinotecan. Pharmacogenetics 13:741–757
Zhou Q, Sparreboom A, Tan EH, Cheung YB, Lee A, Poon D, Lee EJ, Chowbay B (2005) Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol 59:415–424
Innocenti F, Undevia SD, Chen PX, Das S, Ramirez J, Dolan ME, Relling MV, Kroetz DL, Ratain MJ (2004) Pharmacogenetic analysis of interindividual irinotecan (CPT-11) pharmacokinetic (PK) variability: evidence for a functional variant of ABCC2. In: 2004 ASCO annual meeting proceedings (post-meeting edition), vol 22, No 14S, abstract no: 2010
de Jong FA, Marsh S, Mathijssen RH, King C, Verweij J, Sparreboom A, McLeod HL (2004) ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res 10:5889–5894
de Jong FA, Scott-Horton TJ, Kroetz DL, McLeod H, Friberg LE, Mathijssen RH, Verweij J, Marsh S, Sparreboom A (2007) Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther 81:42–49
Xiang X, Jada SR, Li HH, Fan L, Tham LS, Wong CI, Lee SC, Lim R, Zhou QY, Goh BC, Tan EH, Chowbay B (2006) Pharmacogenetics of SLCO1B1 gene and the impact of *1b and *15 haplotypes on irinotecan disposition in Asian cancer patients. Pharmacogenet Genomics 16:683–691
Takane H, Miyata M, Burioka N, Kurai J, Fukuoka Y, Suyama H, Shigeoka Y, Otsubo K, Ieiri I, Shimizu E (2007) Severe toxicities after irinotecan-based chemotherapy in a patient with lung cancer: a homozygote for the SLCO1B1*15 allele. Ther Drug Monit 29:666–668
Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Kim HT, Lee JS (2008) Influence of the organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on irinotecan-pharmacokinetics and clinical outcome of patients with advanced non-small cell lung cancer. Lung Cancer 59:69–75
Han JY, Lim HS, Park YH, Lee SY, Lee JS (2009) Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 63:115–120
Michael M, Thompson M, Hicks RJ, Mitchell PL, Ellis A, Milner AD, Di Iulio J, Scott AM, Gurtler V, Hoskins JM, Clarke SJ, Tebbut NC, Foo K, Jefford M, Zalcberg JR (2006) Relationship of hepatic functional imaging to irinotecan pharmacokinetics and genetic parameters of drug elimination. J Clin Oncol 24:4228–4235
Sai K, Itoda M, Saito Y, Kurose K, Katori N, Kaniwa N, Komamura K, Kotake T, Morishita H, Tomoike H, Kamakura S, Kitakaze M, Tamura T, Yamamoto N, Kunitoh H, Yamada Y, Ohe Y, Shimada Y, Shirao K, Minami H, Ohtsu A, Yoshida T, Saijo N, Kamatani N, Ozawa S, Sawada J (2006) Genetic variations and haplotype structures of the ABCB1 gene in a Japanese population: an expanded haplotype block covering the distal promoter region, and associated ethnic differences. Ann Hum Genet 70:605–622
Sai K, Saito Y, Itoda M, Fukushima-Uesaka H, Nishimaki-Mogami T, Ozawa S, Maekawa K, Kurose K, Kaniwa N, Kawamoto M, Kamatani N, Shirao K, Hamaguchi T, Yamamoto N, Kunitoh H, Ohe Y, Yamada Y, Tamura T, Yoshida T, Minami H, Matsumura Y, Ohtsu A, Saijo N, Sawada J (2008) Genetic variations and haplotypes of ABCC2 encoding MRP2 in a Japanese population. Drug Metab Pharmacokinet 23:139–147
Maekawa K, Itoda M, Sai K, Saito Y, Kaniwa N, Shirao K, Hamaguchi T, Kunitoh H, Yamamoto N, Tamura T, Minami H, Kubota K, Ohtsu A, Yoshida T, Saijo N, Kamatani N, Ozawa S, Sawada J (2006) Genetic variation and haplotype structure of the ABC transporter gene ABCG2 in a Japanese population. Drug Metab Pharmacokinet 21:109–121
Kim SR, Saito Y, Sai K, Kurose K, Maekawa K, Kaniwa N, Ozawa S, Kamatani N, Shirao K, Yamamoto N, Hamaguchi T, Kunitoh H, Ohe Y, Yamada Y, Tamura T, Yoshida T, Minami H, Ohtsu A, Saijo N, Sawada J (2007) Genetic variations and frequencies of major haplotypes in SLCO1B1 encoding the transporter OATP1B1 in Japanese subjects: SLCO1B1*17 is more prevalent than *15. Drug Metab Pharmacokinet 22:456–461
Takane H, Kobayashi D, Hirota T, Kigawa J, Terakawa N, Otsubo K, Ieiri I (2004) Haplotype-oriented genetic analysis and functional assessment of promoter variants in the MDR1 (ABCB1) gene. J Pharmacol Exp Ther 311:1179–1187
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR (2001) Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 70:189–199
Choi JH, Ahn BM, Yi J, Lee JH, Lee JH, Nam SW, Chon CY, Han KH, Ahn SH, Jang IJ, Cho JY, Suh Y, Cho MO, Lee JE, Kim KH, Lee MG (2007) MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet Genomics 17:403–415
Tirona RG, Leake BF, Merino G, Kim RB (2001) Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276:35669–35675
Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ, Eichelbaum M, Kivistö KT (2004) High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics 14:429–440
Saeki M, Saito Y, Sai K, Maekawa K, Kaniwa N, Sawada J, Kawamoto M, Saito A, Kamatani N (2007) A combinatorial haplotype of the UDP-glucuronosyltransferase 1A1 gene (#60-#IB) increases total bilirubin concentrations in Japanese volunteers. Clin Chem 53:356–358
Tamura A, Wakabayashi K, Onishi Y, Takeda M, Ikegami Y, Sawada S, Tsuji M, Matsuda Y, Ishikawa T (2007) Re-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2. Cancer Sci 98:231–239
Wong M, Evans S, Rivory LP, Hoskins JM, Mann GJ, Farlow D, Clarke CL, Balleine RL, Gurney H (2005) Hepatic technetium Tc 99m-labeled sestamibi elimination rate and ABCB1 (MDR1) genotype as indicators of ABCB1 (P-glycoprotein) activity in patients with cancer. Clin Pharmacol Ther 77:33–42
Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, Mielke S (2006) Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer 42:2893–2896
Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y (2002) C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 1:611–616
Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y (2004) Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res 21:1895–1903
Mizuarai S, Aozasa N, Kotani H (2004) Single nucleotide polymorphisms result in impaired membrane localization and reduced ATPase activity in multidrug transporter ABCG2. Int J Cancer 109:238–246
Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C, Verweij J, McLeod HL (2004) Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther 76:38–44
Teng S, Piquette-Miller M (2008) Regulation of transporters by nuclear hormone receptors: implications during inflammation. Mol Pharm 5:67–76
Englund G, Jacobson A, Rorsman F, Artursson P, Kindmark A, Rönnblom A (2007) Efflux transporters in ulcerative colitis: decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm Bowel Dis 13:291–297
de Jong F, van der Bol J, Mathijssen R, van Gelder T, Wiemer E, Sparreboom A, Verweij J (2008) Renal function as a predictor of irinotecan-induced neutropenia. Clin Pharmacol Ther 84:254–262
Acknowledgments
This study was supported in part by the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and by the Program for the Promotion of Studies in Health Sciences of the Ministry of Health, Labor and Welfare of Japan. We thank Yakult Honsha Co., Ltd (Tokyo, Japan) for providing analytical standards of irinotecan and its metabolites. We also thank Ms. Chie Sudo for her administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sai, K., Saito, Y., Maekawa, K. et al. Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother Pharmacol 66, 95–105 (2010). https://doi.org/10.1007/s00280-009-1138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1138-y