Abstract
In heart failure, intracellular Ca2+ leak from cardiac ryanodine receptors (RyR2s) leads to a loss of Ca2+ from the sarcoplasmic reticulum (SR) potentially contributing to decreased function. Experimental data suggest that the 1,4-benzothiazepine K201 (JTV-519) may stabilise RyR2s and thereby reduce detrimental intracellular Ca2+ leak. Whether K201 exerts beneficial effects in human failing myocardium is unknown. Therefore, we have studied the effects of K201 on muscle preparations from failing human hearts. K201 (0.3 μM; extracellular [Ca2+]e 1.25 mM) showed no effects on contractile function and micromolar concentrations resulted in negative inotropic effects (K201 1 μM; developed tension −9.8 ± 2.5% compared to control group; P < 0.05). Interestingly, K201 (0.3 μM) increased the post-rest potentiation (PRP) of failing myocardium after 120 s, indicating an increased SR Ca2+ load. At high [Ca2+]e concentrations (5 mmol/L), K201 increased PRP already at shorter rest intervals (30 s). Strikingly, treatment with K201 (0.3 μM) prevented diastolic dysfunction (diastolic tension at 5 mmol/L [Ca2+]e normalised to 1 mmol/L [Ca2+]e: control 1.26 ± 0.06, K201 1.01 ± 0.03, P < 0.01). In addition at high [Ca2+]e, K201 (0.3 μM) treatment significantly improved systolic function [developed tension +27 ± 8% (K201 vs. control); P < 0.05]. The beneficial effects on diastolic and systolic functions occurred throughout the physiological frequency range of the human heart rate from 1 to 3 Hz. Upon elevated intracellular Ca2+ concentration, systolic and diastolic contractile functions of terminally failing human myocardium are improved by K201.
Similar content being viewed by others
Introduction
Patients with chronic heart failure (HF) exhibit depressed contractile function and ventricular arrhythmias, which may result in sudden death. Abnormal intracellular Ca2+ handling is a likely mechanism contributing to decreased force development and has been associated with impaired SR Ca2+ storage function [3, 4, 15]. Luminal SR Ca2+ concentrations are determined by diastolic SR Ca2+ uptake through ATPases (SERCA2s) as well as diastolic Ca2+ leak via ryanodine receptors (RyR2s). Although the dynamic leak-load relationship may limit SR Ca2+ loss resulting from decreased SERCA2 function in HF [23], chronically increased RyR2 open probability may impair contractile performance [27] and may also lead to triggered arrhythmias [24].
Recently, novel pharmacological and genetic therapeutic strategies were developed which target abnormal intracellular Ca2+ cycling in HF and arrhythmias [5, 13, 22]. Among these, the 1,4-benzothiazepine compound K201 (also known as JTV-519 [9]) has shown beneficial effects in animals with HF [28, 31] or arrhythmias [26]. K201 has multiple sites of action in the heart. The most interesting action of K201 is the ability to induce a conformational change in RYR reducing its open probability [30]. Other actions of K201 are the inhibition of SERCA [14] and several sarcolemmal ion-channels [10]. These different actions were demonstrated to depend on the concentration of K201 and concentrations up to 1 μM K201 are used to ensure RYR-mediated effects [12].
To investigate the efficacy of K201 to improve human cardiac muscle function, intact muscle preparations form terminally failing human hearts were used, and the force frequency response (FFR) and post-rest potentiation (PRP) protocols were applied to study SR-dependent muscle performance [18]. In particular, PRP has been shown to correlate with SR Ca2+ content [19]. Our data identify potentially beneficial K201 effects on contractile function in terminally failing human hearts.
Methods
Muscle preparation
Human ventricular muscle strips were dissected from freshly explanted hearts of 14 end-stage heart failure patients undergoing cardiac transplantation as a result of ischemic or dilated cardiomyopathy (12 men and 2 women, average age 42.2 ± 4.2 years). Unfortunately, the analysis of non-failing myocardium was not possible due to the lack of available tissue. Detailed patient characteristics are provided in the online supplement. The investigation conforms to the principles outlined in the Declaration of Helsinki. The study was approved by the institutional ethics committee, and all patients provided written informed consent for the use of cardiac tissue samples. Hearts were transported in a Krebs–Henseleit buffer (KHB) with 2,3-butanedione monoxime as cardioplegic solution [8]. Intact trabeculae were carefully microdissected from the left ventricle and fixed between a force transducer (Scientific Instruments) and a hook connected to a micromanipulator for length adjustment. Only trabeculae with a diameter of 0.5 mm or less were used for experiments. Mean dimensions (mm) were as follows: diameter 1 0.40 ± 0.01; diameter 2 0.36 ± 0.01; length 2.2 ± 0.1. The distribution of the muscle diameter was not different between the analysed groups. After wash-out of the cardioplegic solution, muscle preparations were superfused with Krebs–Henseleit solution (containing in mmol/L: 137 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 20 HEPES, 10 glucose, 0.25 CaCl2; pH adjusted to 7.4 with NaOH) and electrically stimulated (baseline 1 Hz, amplitude 3–5 V; stimulator Scientific Instruments type STIM2). Force measurements were carried out at 37°C and either at 1.25 or 5 mmol/L [Ca2+]o. After 60 min of equilibration, the muscles were stretched gradually to the length at which maximum steady-state twitch force was reached (L max).
Experimental protocol
K201 was a gift from Aetas Pharma Ltd. (Japan). The drug was dissolved in DMSO (99.7%, Sigma-Aldrich, Taufkirchen, Germany) to achieve a nominal stock concentration of 1 mmol/L. To determine the effect of K201 on basal contractile performance, K201 was added to the circulating KHB solution in different concentrations (0.1, 0.3, 1, 3, 10, 30 μM) and compared to the control group treated with the drug carrier (DMSO, same vol% dilution corresponding to each K201 step). The developed tension (T dev), diastolic tension (T dia), time to peak (TTP) and time to 50 and 90% relaxation (RT50 and RT90) were recorded online using LabView (National instruments).
In all further experiments (Fig. 1), K201 was added to the solution at least 60 min before assessment of muscles strip function. Force-frequency relationship and PRP were analysed in K201 (0.3 or 1 μM) treated preparations. The FFR was examined at a range of 0.25–3 Hz and normalised to 0.25 Hz. Force recording of isometric tension was obtained at steady-state conditions at each frequency step. PRP was examined using rest intervals of 1–120 s (1, 2, 4, 8, 16, 30, 60, 120 s). The tension developed on the first twitch after rest was divided by the mean developed tension of the last ten beats before rest.
To provoke an increase in SR Ca2+ leak and diastolic tension extracellular calcium ([Ca2+]e) was elevated stepwise from 1.25 to 5 mmol/L [1, 2.] Upon steady state, PRP protocol was conducted as described above to ascertain equal starting conditions in all groups. Then the muscles were randomised to treatment with either K201 at 0.3 or 1 μM or DMSO and left contracting for 2 h. After 2 h the PRP was repeated again.
To examine the influence of calcium on diastolic tension muscles were treated with 0.3 μM K201 or DMSO at the start of the experiment and stretched to L max at 1.0 mmol/L [Ca2+]e. Upon steady state the [Ca2+]e was increased stepwise (1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0) to 5 mmol/L. Contractile parameters were analysed after every step. Finally, FFR was measured in these muscles strips with 5 mmol/L [Ca2+]e.
Mean developed tension of failing human myocardial muscle strips at 1 Hz was 12.2 ± 2.1 mN/mm2.
Calculation and statistics
Tension measurements were normalised to the cross-sectional area for each preparation, calculated assuming an elliptical cross section using the formula cross-sectional area = D 1/2 × D 2/2 × π, with D1 and D2 representing width and thickness, and expressed as tension. Determined parameters were expressed as the ratio of the respective baseline parameter. Contractile parameters at different K201 concentrations were analysed using using two-way ANOVA or paired Students t test where appropriate, with values of P < 0.05 considered statistically significant.
Results
Concentration-dependent response to K201
Compared to control (n = 6), developed tension (T dev) remained unchanged until 0.3 μM K201 and declined at a concentration of 1 μM (1 μM: 9.8 ± 2.5% of control, P < 0.05, n = 6, Fig. 2). Higher doses of K201 lead to a further reduction of T dev. At 30 μM the developed tension was reduced to only 23 ± 5% (P < 0.001) of control. The half-maximal inhibitory concentration (IC50) of K201 on the developed tension was 8.7 μM (Supplementary figure 1).
Effects of K201 on contractile performance at low [Ca2+]e in failing human hearts
Isolated ventricular muscle preparations from terminally failing human hearts did not show an acutely improved contractile function following K201 at 0.3 μM and clearly K201 may be acutely negatively inotropic at high concentrations (Fig. 2; Supplementary figure 1). However, K201 has a profound effect on post rest potentiation even at concentrations of 0.3 μM, where contractility is largely preserved (Fig. 3a). In human failing cardiac muscle strips we typically observe a robust post rest potentiation (Fig. 3b). Up to 16 s the increase in post rest potentiation was similar between K201 or control treated muscle strips (increase from 0 to 16 s: control: 93 ± 17%; 0.3 μM K201: 87 ± 12%; 1 μM K201: 94 ± 11%, each P < 0.01, Fig. 3b). At longer rest intervals, a further increase in post rest potentiation was visible in the K201 treated muscles strips (increase from 16 to 120 s: 0.3 μM K201: 59 ± 27%; 1 μM K201: 62 ± 34%, each P < 0.05), but not in the control group (increase from 16 to 120 s: 7 ± 14%, P = 0.61, Fig. 3c).
Effect of 0.3 μM (dotted line, n = 6) and 1 μM K201 (dashed line, n = 6) and control (solid line, n = 6) on PRP at 1.25 mmol/L [Ca2+]e (a). Increase of post rest potentiation at 16 s normalised to 0 s (b) and at 120 s normalised to 16 s (c) in control (black bar) and 0.3 μM (light gray bar) or 1 μM (dark grey bar) K201. *P < 0.05, $ P < 0.01
Additionally, we observed a frequency-dependent significant increase in diastolic tension and a blunted increase in systolic tension in failing human myocardium. However, both diastolic and systolic FFR were not significantly altered by either K201 treatement (0.3 or 1 μM) compared to control (Supplementary figure 2). These results are in agreement with earlier data from failing human myocardium demonstrating that the phenotype of increased diastolic tension in combination with a blunted FFR is associated with decreased SERCA2 and increased NCX function, further contributing to depletion of intracellular Ca2+ stores through competitive mechanisms and explaining the relatively long PRP needed to observe beneficial efficacy of K201 earlier.
Influence of K201 treatment on PRP behaviour at elevated [Ca2+]e in human failing hearts
Since our initial data indicated that K201 effects on PRP behaviour required relatively long rest intervals under relatively Ca2+ deprived conditions, we investigated PRP before and after prolonged (2 h) treatment with 0.3 or 1 μM K201 (Fig. 4a, b). Before treatment PRP was not different between the experimental groups (Fig. 4c). However, PRP was significantly increased by 38 and 35% using 0.3 or 1 μM K201, respectively (normalised increase after a rest interval of 120 s: 1.68 ± 0.12 in the control group and 2.32 ± 0.24 and 2.28 ± 0.20 in the K201 group with a concentration of 0.3 and 1 μM, respectively P < 0.05, Fig. 4d).
Representative examples of a PRP (a) before (black) and 2 h after (grey) treatment with K201 at 5 mmol/L [Ca2+]e. The dashed line shows the maximal increase of post rest potentiation of the untreated muscles strip. Example of post rest contractile performance (b) after 30 s rest interval in control (black) and 0.3 μM K201 (dashed line). Comparison of the effect of 0.3 μM (dotted line, n = 7) or 1 μM K201 (dashed line, n = 7) and control (solid line, n = 7) on PRP at 5 mmol/L [Ca2+]e before (c) and 2 h after (d) treatment with K201 or control. *P < 0.05, $ P < 0.05
Effect of K201 on contractile performance during elevation of [Ca2+]e in human failing hearts
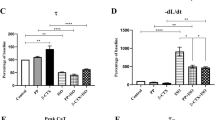
Since our initial data indicated that K201 effects on PRP behaviour required relatively long rest intervals under relatively Ca2+ deprived conditions, we investigated failing ventricular preparations at supra-physiological [Ca2+]e concentrations. Above normal [Ca2+]e can be expected to lead to a relative increase in SR intraluminal Ca2+ concentrations [20]; however, the pre-existing competition between SERCA2 and NCX can be expected to partially extrude the additional and potentially inotropic Ca2+ to the extracellular side. Therefore, K201 by inhibiting SR Ca2+ leak may improve SR Ca2+ load independently from pre-existing SERCA2 and NCX mechanisms. As expected, increased [Ca2+]e (5 mmol/L) resulted in a significant increase in diastolic tension (Fig. 5a) of +25.7 ± 5.9% (relative to baseline conditions at 1 mmol/L; P < 0.05, each n = 7; Fig. 4c). However, K201 treatment (0.3 μM) abolished the increase in diastolic tension (n = 7, P < 0.001, Fig. 5b, c). Additionally, following the same K201 treatment developed tension (T dev) was significantly increased by +27 ± 8% (P < 0.05; Fig. 5c) at 5 mmol/L [Ca2+]e. In agreement with earlier reports [16], myocardial relaxation was significantly prolonged at higher Ca2+ concentrations in control preparations (RT90 1.0 vs. 5.0 mmol/L [Ca2+]e: +19 ± 7%, P < 0.05). However, K201 treatment prevented the increase in relaxation time (RT90 1.0 vs. 5.0 mmol/L [Ca2+]e: +14 ± 10%, not significant). Thus, our data indicate that K201 exerts beneficial effects including myocardial contraction and relaxation in failing human myocardium.
Original recordings from two experiments during the increase of [Ca2+]e from 3.0 to 5.0 mmol/L from control (a) or with 0.3 μM K201 (b) muscles strip experiments. c Effect of [Ca2+]e on the diastolic (open symbols) and developed tension (filled symbols) of human failing muscle strips treated with either 0.3 μM K201 (dotted line, n = 7) or control (solid line, n = 7). Tension is expressed relative to tension at [Ca2+]e of 1 mmol/L, *P < 0.05
Influence of K201 on the FFR at elevated [Ca2+]e in human failing hearts
Increasing the stimulation frequency in muscles strips with elevated [Ca2+]e leads to a frequency-dependent decline in developed tension and an increase in diastolic tension. Taking into account that the contractile performance at 1 Hz was already different in the K201-treated group compared to control, the frequency-dependent changes in developed and diastolic tension were normalised to the tension at 1 Hz and 1.0 mmol/L [Ca2+]e (Fig. 6a, c). The contractile improvement by K201 was preserved also at higher frequencies (Fig. 6b, d). The developed tension was higher in the K201-treated muscles strips at the stimulation frequencies from 1 to 3 Hz (K201 vs. control: 1 Hz: 2.99 ± 0.25 vs. 2.35 ± 0.11; 2 Hz: 1.50 ± 0.23 vs. 0.89 ± 0.14; 3 Hz: 0.65 ± 0.09 vs. 0.39 ± 0.06; each n = 7 and P < 0.05, Fig. 6b). Also the diastolic tension was lower throughout the whole rage of the FFR (K201 vs. control: 1 Hz: 1.01 ± 0.03 vs. 1.25 ± 0.06; 2 Hz: 1.69 ± 0.35 vs. 3.07 ± 0.69; 3 Hz: 2.13 ± 0.47 vs. 3.75 ± 0.75; each n = 7 and P < 0.05, Fig. 6d).
After elevation of the [Ca2+]e to 5.0 mmol/L (a, c) the frequency was increased from 1 to 2 and 3 Hz (b, d). The frequency-dependent changes of human failing muscles strips treated with either 0.3 μM K201 (dotted line, n = 7) or control (solid line, n = 7) in developed (b) and diastolic tension (d) were normalised to the tension at 1.0 mmol/L [Ca2+]e and 1 Hz taking into account, that the contractile performance at 5.0 mmol/L [Ca2+]e is already different in the K201-treated muscles strips compared to controls. P < 0.05
Discussion
Our data suggest that the 1,4-benzothiazepine K201 improves both diastolic and systolic contractile function of failing human myocardium. The following observations support these conclusions: (1) PRP ([Ca2+]e 1.25 mM) was improved by K201 at longer post-rest intervals; (2) K201 ([Ca2+]e 5 mM) improved PRP at shorter post-rest intervals; (3) K201 prevented diastolic dysfunction and improved systolic function under Ca2+ overload conditions; (4) the beneficial effects on systolic and diastolic function were maintained throughout the physiological frequency range of the human heart.
Importantly, systolic improvement is confined to low concentrations of K201 as higher concentrations are clearly negatively inotropic.
Apart from the potentially therapeutic effects of K201 on RyR2 dysfunction and intracellular Ca2+ leak [9, 11, 12], K201 has documented off-target effects at high micromolar concentrations. Therefore, the negative inotropic effect on developed tension in failing human myocardial preparations may be attributed to inhibitory actions of micromolar K201 concentrations on L-type Ca2+ currents and SERCA [10, 14]. However, at nanomolar concentrations of K201 (0.3 μM), no adverse effects on the contractile performance of failing human myocardium were observed. Additionally, at low physiological [Ca2+]e contractile function was not affected by K201 treatment in failing muscle preparation. This finding is in agreement with studies showing that SR Ca2+ leak depends on sufficient SR Ca2+ load [29] and that extracellular Ca2+-depletion leads to reduced SR Ca2+ load and reduced SR Ca2+ leak [23]. Thus, our data under low [Ca2+]e conditions agree with the hypothesis that K201 targets SR Ca2+ leak but, importantly, does not block physiological SR Ca2+ release and therefore exerts no beneficial or adverse effects. Additionally, K201 showed significant beneficial effects on PRP at longer post-rest intervals which have been associated with increased SR Ca2+ load [20]. Again, these data support that K201 effects depend on the existence of significant SR Ca2+ leak in human failing muscle strips due to longer SR Ca2+ load periods.
Kimura et al. [10] showed that the half-maximal inhibitory concentration (IC50) of K201 on plasma membrane ion currents is 5 μM. Loughrey et al. [14] showed that K201 in a concentration of 3 μM significantly reduced SERCA activity. However, we have not observed any adverse changes on FFR behaviour throughout the entire physiological frequency range of the human heart with K201 at 0.3 μM; thus our results may be explained by a up to a magnitude order lower K201 concentrations.
Indeed, experiments with human cardiac muscle preparations have been established as a solid model of cardiac excitation–contraction-coupling. For example, it has been shown that the FFR in isolated muscle preparations resembles the FFR in patients with HF [6], and that intracellular Ca2+ cycling is depressed in muscle preparations following post-rest activation [20]. Accordingly, we have used increased [Ca2+]e as a model of increased intracellular Ca2+ load in accordance with previously established protocols [1, 2] to characterise potential beneficial effects of K201 under conditions of increased SR Ca2+ leak as shown earlier [14]. As we did not measure Ca2+ directly, our study relies on previous observations showing that SR Ca2+ leak is a function of SR Ca2+ load [29] and that SR Ca2+ concentration in intact cardiac myocytes depends directly on intracellular Ca2+ concentration [23]. Importantly, an increase in intracellular Ca2+ concentration was achieved by increasing [Ca2+]e as reported earlier in non-human material [1] and was reported to be associated with multiple spontaneous SR Ca2+ release events [14].
Indeed, following K201 treatment, failing human muscle strip preparations showed improved PRP behaviour at shorter post-rest intervals under high [Ca2+]e conditions. These results are in line with previously published animal data [11, 27] and demonstrate beneficial effects of nanomolar concentrations of K201 (0.3 μM). Our results confirm earlier animal studies by Kohno et al. [11] who have found efficacy of K201 against intracellular Ca2+ leak at the same concentration.
A limitation of our study is the lack of direct Ca2+ measurements. Although this was achieved technically in the past, in our hands it is clearly not possible to obtain these measurements at physiological temperature and stimulus rates (whereas the data presented here was measured at 37°C under near physiological conditions).
A significant finding is that K201 improved both diastolic and developed tension throughout the physiological frequency range of the human heart. Indeed, altered FFR is an important pathophysiological mechanism of reduced cardiac performance and altered stress adaptation in human HF [18, 21]. The combined beneficial effects of K201 on diastolic function and the FFR behaviour in failing human myocardium indicate potentially beneficial effects of 1,4-benzothiazepines which are distinct from existing pharmacological strategies.
Previously the mechanism of K201 on RYR2 stabilisation could be further deciphered: Yamamoto et al. [32] found that the K201-binding site on RYR2 is the domain (2114–2149). Interdomain interaction of RyR2 becomes loose in failing hearts, resulting in SR Ca leak [17, 25]. Addition of K201 to the destabilised RyR2 restores a stable configuration, i.e. inter-domain interaction, as in RyR2 from non-failing myocardium [32]. Rebinding of FKBP12.6 to RyR2 in failing SR seems not to be required to induce the K201 effects on RYR2 [7, 17], but K201 facilitates rebinding of FKBP12.6 in some cases [26, 30], suggesting that K201 may act primarily by modifying domain–domain interaction, but less via FKBP12.6.
We observed a negative inotropic effects at higher concentrations of K201 suggesting a detrimental effect on cardiac performance in failing human hearts. Although this finding does not necessarily imply an unfavourable effect in heart failure (i.e. β-blocker therapy paradoxically improves cardiac function in heart failure), we suggest K201 should be used at concentrations lower than 1 μM when initially examined in humans. Our findings suggest that at such low concentrations there is already a profound effect on calcium cycling in human myocytes without negative inotropy. This gives support to the concept that the antiarrhythmic effects evident in multiple animal models at these lower K201 concentrations will be also present in humans.
In summary, this study demonstrates that K201 improved some aspects of the contractile performance in the human failing myocardium at lower concentrations (0.3 μM) consistent with reduced SR calcium leak. Therefore K201 may, in addition to previously reported anti-arrhythmic effects, improve contractile performance in the failing human heart.
References
Allen DG, Eisner DA, Orchard CH (1984) Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol 350:615–630
Allen DG, Eisner DA, Pirolo JS, Smith GL (1985) The relationship between intracellular calcium and contraction in calcium-overloaded ferret papillary muscles. J Physiol 364:169–182
Bers DM (2001) Excitation–contraction coupling and cardiac contractile force, 2nd edn. Kluwer, Dordrecht
Bøkenes J, Aronsen JM, Birkeland JA, Henriksen UL, Louch WE, Sjaastad I, Sejersted OM (2008) Slow contractions characterize failing rat hearts. Basic Res Cardiol 103(4):328–344
Györke S, Terentyev D (2008) Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77:245–255
Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H (1994) Influence of the force–frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J 15:164–170
Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR (2007) K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J 404(3):431–438
Janssen PM, Hasenfuss G, Zeitz O, Lehnart SE, Prestle J, Darmer D, Holtz J, Schumann H (2001) Load-dependent induction of apoptosis in multicellular myocardial preparations. Am J Physiol Heart Circ Physiol 282:H349–H356
Kaneko N (1994) New 1, 4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res 33:429–438
Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H (1999) Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol 79:275–281
Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, Okuda S, Ohkusa T, Kohno M, Matsuzaki M (2003) A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol Heart Circ Physiol 284:H1035–H1042
Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR (2006) Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA 103:7906–7910
Lehnart SE (2007) Novel targets for treating heart, muscle disease: stabilizing ryanodine receptors, preventing intracellular calcium leak. Curr Opin Pharmacol 7:225–232
Loughrey CM, Otani N, Seidler T, Craig MA, Matsuda R, Kaneko N, Smith GL (2007) K201 modulates excitation–contraction coupling and spontaneous Ca2+ release in normal adult rabbit ventricular cardiomyocytes. Cardiovasc Res 76:236–246
Maack C, O’Rourke B (2007) Excitation–contraction coupling and mitochondrial energetics. Basic Res Cardiol 102(5):369–392
Miura DS, Biedert S, Barry WH (1981) Effects of calcium overload on relaxation in cultured heart cells. J Mol Cell Cardiol 13:949–961
Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, Matsuzaki M (2005) Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation 111(25):3400–3410
Pieske B, Hasenfuss G, Holubarsch C, Schwinger R, Böhm M, Just H (1992) Alterations of the force–frequency relationship in the failing human heart depend on the underlying cardiac disease. Basic Res Cardiol 87(Suppl 1):213–221
Pieske B, Maier LS, Bers DM, Hasenfuss G (1999) Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85:38–46
Pieske B, Sütterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G (1996) Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest 98:764–776
Schillinger W, Lehnart SE, Prestle J, Preuss M, Pieske B, Maier LS, Meyer M, Just H, Hasenfuss G (1998) Influence of SR Ca(2+)-ATPase and Na(+)-Ca(2+)-exchanger on the force–frequency relation. Basic Res Cardiol 93(Suppl 1):38–45
Seidler T, Hasenfuss G, Maier LS (2007) Targeting altered calcium physiology in the heart: translational approaches to excitation, contraction, and transcription. Physiology (Bethesda) 22:328–334
Shannon TR, Ginsburg KS, Bers DM (2002) Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91:594–600
Shannon TR, Pogwizd SM, Bers DM (2003) Elevated sarcoplasmic reticulum Ca2+-leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93:592–594
Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M (2009) Defective domain–domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res 81(3):536–545
Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304(5668):292–296
Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Morales R, Sun J, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR (2005) Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA 102:9607–9612
Wehrens XH, Reiken S, Vest JA, Wronska A, Marks AR (2006) Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA 103:511–518
Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR (2007) Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem 282:30256–30264
Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M (2003) FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107:477–484
Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Noguchi N, Takasawa S, Okamoto H, Matsuzaki M (2000) Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation 102:2131–2136
Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, Oda T, Kobayashi S, Ikemoto N, Matsuzaki M (2008) Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation 117(6):762–772
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft [grant HA 1233/7-3 to (T.S. and G.H.), grant KFO 155 TP1 (G.H., 1873/2-1), TP3 (T.S.) and TP4 (S.E.L.)], EUGeneHeart (project number LSHM-CT-2005-018833). S.E.L. is a Alfried Krupp von Bohlen and Halbach Foundation endowed Professor of Translational Cardiology.
Conflict of interest statement
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Toischer, K., Lehnart, S.E., Tenderich, G. et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol 105, 279–287 (2010). https://doi.org/10.1007/s00395-009-0057-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0057-8