Abstract
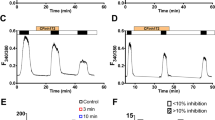
The cAMP-dependent activation of the cystic fibrosis transmembrane conductance regulator (CFTR) and its modulation through inhibition of phosphodiesterases (PDE) were studied with the cell-attached patch-clamp technique in Calu-3 cells (expressing endogenous CFTR) and NIH3T3 cells [expressing either wild-type (Wt)-CFTR or ΔF508-CFTR]. In Calu-3 cells, CFTR current was augmented by increasing concentrations of 8-(4-chlorophenylthio)-adenosine 3′,5′-cyclic monophosphate (CPT–cAMP) and reached a saturating level at ≥60 µM. Varying the forskolin concentration also modulated CFTR activity; 10 µM was maximally effective since supplemental application of 200 µM CPT–cAMP had no additional effect. Activation of CFTR by increasing the cAMP concentration occurs through an increase of the NP o (product of the number of functional channels and the open probability) since the single-channel amplitude remains unchanged. In Calu-3 and NIH3T3-Wt cells, PDE inhibitors, milrinone (100 µM), 8-cyclopentyl-1,3-dipropylxanthine (CPX, 25 µM), and 3-isobutyl-1-methylxanthine (IBMX, 200 µM), did not enhance CFTR current initially activated with 10 µM forskolin, but each potentiated CFTR activity elicited with a submaximal forskolin concentration (e.g., 100 nM) and prolonged the deactivation of CFTR channel current upon removal of forskolin. Millimolar IBMX increased the NP o of both Wt- and ΔF508-CFTR even under maximal cAMP stimulation. Quantitatively, these effects of millimolar IBMX on NP o approximate those of genistein, which potentiates the cAMP-dependent CFTR activity via a mechanism that does not involve increases in cellular cAMP. Thus, depending on the concentration, PDE inhibitors may affect CFTR through different mechanisms.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 October 1998 / Accepted: 10 November 1998
Rights and permissions
About this article
Cite this article
Al-Nakkash, L., Hwang, TC. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflügers Arch 437, 553–561 (1999). https://doi.org/10.1007/s004240050817
Issue Date:
DOI: https://doi.org/10.1007/s004240050817