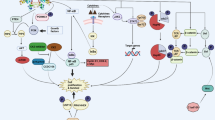
Protein kinase CK2 is a highly conserved enzyme composed of two catalytic subunits α and/or α′ and two regulatory subunits β whose activity is elevated in diverse tumour types as well as in highly proliferating tissues. Several results suggest that the overexpression of either CK2 catalytic subunits or the CK2 holoenzyme contributes to cellular transformation. In a similar vein, experiments performed compromising the intracellular expression of CK2 has led to somehow contradictory results with respect to the ability of this enzyme to control survival and apoptosis. To better elucidate the role of CK2 in programmed cell death, we have depleted cells of CK2 catalytic subunits by the application of antisense oligodeoxynucleotides and siRNAs techniques, respectively. Our results indicate that protein kinase CK2 is characterized by an extremely high stability that might be due to its association with other intracellular proteins, enhanced half-life or lower vulnerability towards proteolytic degradation. In addition, we show that despite the effectiveness of the methods applied in lowering CK2 kinase activity in all cells investigated, CK2 might not by itself be sufficient to trigger enhanced drug-induced apoptosis in cells.
Similar content being viewed by others
References
Faria M, Spiller DG, Dubertret C, et al. Phosphoramidate oligonucleotides as potent antisense molecules in cells and in vivo. Nature 2001; 19: 40–44.
Cerutti H. RNA interference: travelling in the cell and gaining functions? Trends in Gen 2003; 19: 39–46.
Guerra B, Issinger O-G. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 1999; 20: 391–408.
Cochet C, Chambaz EM. Oligomeric structure and catalytic activity of G type casein kinase. Isolation of the two subunits and renaturation experiments. J Biol Chem 1983; 258: 1403–1406.
Yang-Feng TL, Naiman T, Kopatz I, Eli D, Dafni N, Canaani D. Assignment of the human casein kinase II alpha’ subunit gene (CSNK2A1) to chromosome 16p13.2-p13.3. Genomics 1994; 19: 173.
Wirkner U, Voss H, Lichter P, Ansorge W, Pyerin W. The human gene (CSNK2A1) coding for the casein kinase II subunit alpha is located on chromosome 20 and contains tandemly arranged Alu repeats. Genomics 1994; 19: 257–265.
Litchfield DN, Luescher B. Casein kinase II in signal transduction and cell cycle regulation. Mol Cell Biochem 1993; 127/128: 187–199.
Glover CV 3rd. On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Bio 1998; 59: 95–133.
Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet 1999; 23(1): 118–121.
Graham KC, Litchfield DW. The regulatory beta subunit of protein kinase CK2 mediates formation of tetrameric CK2 complexes. J Biol Chem 2000; 275: 5003–5010.
Valero E, De Bonis S, Filhol O, et al. Quaternary structure of casein kinase 2. Characterization of multiple oligomeric states and relation with its catalytic activity. J Biol Chem 1995; 270: 8345–8352.
Faust M, Montenarh M. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res 2000; 301: 329–340.
Seitz G, Muenstermann U, Schneider HR, Issinger O-G. Characterization of casein kinase II in human colonic carcinomas after heterotransplantation into nude mice. Biochem Biophys Res Commun 1989; 163: 635–641.
Pistorius K, Seitz G, Remberger K, Issinger O-G. Differential CKII activities in human colorectal mucosa, adenomas and carcinomas. Onkologie 1991; 14: 256–260.
Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 1995; 267: 894–897.
Kelliher MA, Seldin DC Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase II alpha. EMBO J 1996; 15: 5160–5166.
Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2α collaborate in the development of thymic lymphomas in mice. Oncogene 1998; 16: 2965–2974.
Orlandini M, Semplici F, Ferruzzi R, Meggio F, Pinna LA, Oliviero S. Protein kinase CK2alpha’ is induced by serum as a delayed early gene and cooperates with Ha-ras in fibroblast transformation. J Biol Chem 1998; 273: 21291–21297.
Pepperkok R, Lorenz P, Jakobi R, Ansorge W, Pyerin W. Cell growth stimulation by EGF: inhibition through antisense-oligodeoxynucleotides demonstrates important role of Casein Kinase II. Exp Cell Res 1991; 197: 245–253.
Ulloa L, Diaz-Nido J, Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J 1993; 12: 1633–1640.
Ulloa L, Diaz-Nido J, Avila J. Depletion of catalytic and regulatory subunits of protein kinase CK2 by antisense oligonucleotide treatment of neuroblastoma cells. Cell Mol Neurobiol 1994; 14: 407–414.
Formby B, Stern R. Phosphorylation stabilizes alternatively spliced CD44 mRNA transcripts in breast cancer cells: inhibition by antisense complementary to casein kinase II mRNA. Mol & Cell Biochem 1998; 187: 23–31.
Faust RA, Tawfic S, Davis AT, Bubash LA, Ahmed K. Antisense oligonucleotides against protein kinase CK2-alpha inhibit growth of squamous cell carcinoma of the head and neck in vitro. Head Neck 2000; 22: 341–346.
Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem 2001; 227: 167–174.
Sayed M, Pelech S, Wong C, Marotta A, Salh B. Protein kinase CK2 is involved in G2 arrest and apoptosis following spindle damage in epithelial cells. Oncogene 2001; 20: 6994–7005.
Li P-F, Jincheng L, Mueller E-C, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: A signalling switch for the caspase-inhibiting protein ARC. Mol Cell 2002; 10: 247–258.
Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene 2002; 21: 5280–5288.
Guerra B, Issinger O-G. p53 and the ribosomal protein L5 participate in high molecular mass complex formation with protein kinase CK2 in murine teratocarcinoma cell line F9 after serum stimulation and cisplatin treatment. FEBS Lett 1998; 434: 115–120.
Ryan K M, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol 2001; 13: 332–337.
Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 2003; 369: 1–15.
Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol 2001; 16: 573–582.
Russou I, Draetta G. The Schizosaccharomyces pombe casein kinase II alpha and beta subunits: evolutionary conservation and positive role of the beta subunit. Mol Cell Biol 1994; 14: 576–586.
Toczyski DP, Galgoczy DJ, Hartwell LH. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell 1997; 90: 1097–1106.
Luescher B, Litchfield DW. Biosynthesis of casein kinase II in lymphoid cell lines. Eur J Biochem 1994; 220: 521–526.
Zhang C, Vilk G, Canton DA, Litchfield D. Phosphorylation regulates the stability of the regulatory CK2β subunit. Oncogene 2002; 21: 3754–3764.
Hui EK, Nayak DP. Role of G protein and protein kinase signalling in influenza virus budding in MDCK cells. J Gen Virol 2002; 83: 3055–3066.
Ravi R, Bedi A. Sensitization of tumour cells to Apo2 Ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res 2002; 62: 4180–4185.
Desagher S, Osen-Sand A, Montessuit S, et al. Phosphorylation of bid by casein kinase I and II regulates its cleavage by caspase 8. Mol Cell 2001; 8: 601–611.
Keller DM, Zeng X, Wang Y, et al. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell 2001; 7: 283–292.
Wang D, Westerheide SD, Hanson JL, Baldwin AS. Tumor necrosis factor alpha-induced phosphorylation of ReIA/p65 on Ser 529 is controlled by casein kinase II. J Biol Chem 2000; 275: 32592–32597.
McElhinny JA, Trushin SA, Bren GD, Chester N, Paya CV. Casein kinase II phosphorylates I kappa B alpha at S-283, S-289, and T-291 and is required for its degradation. Mol Cell Biol 1996; 16: 899–906.
Luescher B, Kuenzel EA, Krebs EG, Eisenman RN. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J 1989; 8: 1111–1119.
Hessenauer A, Montenarh M, Goetz C. Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. Int J Oncol 2003; 22: 1263–1270.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seeber, S., Issinger, O.G., Holm, T. et al. Validation of protein kinase CK2 as oncological target. Apoptosis 10, 875–885 (2005). https://doi.org/10.1007/s10495-005-0380-y
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-0380-y