Abstract
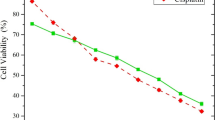
The simultaneous expression of human papillomavirus type 16 (HPV16) E6 and E7 oncogenes is pivotal for malignant transformation and maintenance of malignant phenotypes. Silencing these oncogenes is considered to be applicable in molecular therapies of human cervical cancer. However, it remains to be determined whether HPV16 E6 and E7 could be both silenced to obtain most efficient antitumor activity by using RNA interference (RNAi) technology. Herein, we designed a small interfering RNA (siRNA) targeting HPV16-E7 region to degrade either E6, or truncated E6 (E6*) and E7 mRNAs and to simultaneously knockdown both E6 and E7 expression. Firstly, the sequence targeting HPV16-E7 region was inserted into the shRNA packing vector pSIREN-DNR, yielding pSIREN-16E7 to stably express corresponding shRNA. HPV16-transformed SiHa and CaSki cells were used as a model system; RT-PCR, Western Blotting, MTT assay, TUNEL staining, Annexin V apoptosis assay and flow cytometry were applied to examine the effects of pSIREN-16E7. Our results indicated that HPV16-E7 specific shRNA (16E7-shRNA) induced selective degradation of E6 and E7 mRNAs and proteins. E6 silencing induced accumulation of cellular p53 and p21. In contrast, E7 silencing induced hypophosphorylation of retinoblastoma (Rb) protein. The loss of E6 and E7 reduced cell growth and ultimately resulted in massive apoptotic cell death selectively in HPV-positive cancer cells, compared with the HPV-negative ones. We demonstrated that 16E7-shRNA can induce simultaneous E6 and E7 suppression and lead to striking apoptosis in HPV16-related cancer cells by activating cellular p53, p21 and Rb. Therefore, RNAi using E7 shRNA may have the gene-specific therapy potential for HPV16-related cancers.






Similar content being viewed by others
Reference
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV (2002) The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244–265
Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S (2003) Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 88:63–73
Munoz N, Bosch FX, de Sanjose S et al (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–527
Werness BA, Levine AJ, Howley PM (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76–79
Munger K, Basile JR, Duensing S et al (2001) Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888–7898
Tommasino M, Adamczewski JP, Carlotti F et al (1993) HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene 8:195–202
zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350
He Y, Huang L (1997) Growth inhibition of human papillomavirus 16 DNA-positive mouse tumor by antisense RNA transcribed from U6 promoter. Cancer Res 57:3993–3999
Tan TM, Ting RC (1995) In vitro and in vivo inhibition of human papillomavirus type 16 E6 and E7 genes. Cancer Res 55:4599–4605
Sima N, Wang S, Wang W et al (2007) Antisense targeting human papillomavirus type 16 E6 and E7 genes contributes to apoptosis and senescence in SiHa cervical carcinoma cells. Gynecol Oncol 106:299–304
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Hammond SM, Bernstein E, Beach D, Hannon GJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293–296
Smotkin D, Prokoph H, Wettstein FO (1989) Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol 63:1441–1447
Yoshinouchi M, Yamada T, Kizaki M et al (2003) In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol Ther 8:762–768
Sherman L, Alloul N (1992) Human papillomavirus type 16 expresses a variety of alternatively spliced mRNAs putatively encoding the E2 protein. Virology 191:953–959
Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004) Rational siRNA design for RNA interference. Nat Biotechnol 22:326–330
Jiang M, Milner J (2002) Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene 21:6041–6048
Blagosklonny MV, el-Deiry WS (1996) In vitro evaluation of a p53-expressing adenovirus as an anti-cancer drug. Int J Cancer 67:386–392
el-Deiry WS, Tokino T, Velculescu VE et al (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Darzynkiewicz Z, Bruno S, Del Bino G et al (1992) Features of apoptotic cells measured by flow cytometry. Cytometry 13:795–808
van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1–9
Gu W, Putral L, Hengst K et al (2006) Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther 13:1023–1032
zur Hausen H (1994) Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol 186:131–156
Paddison PJ, Caudy AA, Sachidanandam R, Hannon GJ (2004) Short hairpin activated gene silencing in mammalian cells. Methods Mol Biol 265:85–100
Czauderna F, Santel A, Hinz M et al (2003) Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res 31:e127
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30571950; 30170976; 30672227) and the “973” Program of China (No. 2002CB513100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ni Sima and Wei Wang contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Sima, N., Wang, W., Kong, D. et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of Rb and p53. Apoptosis 13, 273–281 (2008). https://doi.org/10.1007/s10495-007-0163-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0163-8