Abstract
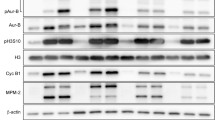
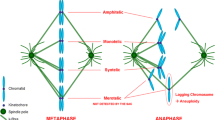
The relationship between microtubular dynamics, dismantling of pericentriolar components and induction of apoptosis was analysed after exposure of H460 non-small lung cancer cells to anti-mitotic drugs. The microtubule destabilising agent, combretastatin-A4 (CA-4) led to microtubular array disorganization, arrest in mitosis and abnormal metaphases, accompanied by the presence of numerous centrosome-independent “star-like” structures containing tubulin and aggregates of pericentrosomal matrix components like γ-tubulin, pericentrin and ninein, whereas the structural integrity of centrioles was not affected by treatment. On the contrary, in condition of prolonged exposure or high concentrations of CA-4 such aggregates never formed. Treatment with 7.5 nM CA-4, which produced a high frequency “star-like” aggregates, was accompanied by mitotic catastrophe commitment characterized by translocation of the proapoptotic Bim protein to mitochondria activation of caspases-3/9 and DNA fragmentation as a result of either prolonged metaphase arrest or attempt of cells to divide. Drug concentrations which fail to block cells at mitosis were also unable to activate apotosis. A detailed time-course analysis of cell cycle arrest and apoptosis indicated that after CA-4 washout the number of metaphases with “star-like” structures decreased as a function of time and arrested cells proceeded in anaphase. After 4 h, the multiple α- and γ-tubulin aggregates coalesced into two well-defined spindles in a bipolar mitotic spindle organization. Overall, our findings suggest that the maintenance of microtubular integrity plays a relevant role in stabilising the pericentriolar matrix, whose dismantling can be associated with apoptosis after exposure to microtubule depolymerising agents.





Similar content being viewed by others
References
Mollinedo F, Gajate C (2003) Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 8:413–450
Islam MN, Iskander MN (2004) Microtubulin binding sites as target for developing anticancer agents. Mini Rev Med Chem 4:1077–1104
Jordan A, Hadfield JA, Lawrence NJ, McGown AT (1998) Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev 18:259–296
Jordan MA, Thrower D, Wilson L (1992) Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci 102:401–416
Wendell KL, Wilson L, Jordan MA (1993) Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-microtubule attachment and in centrosomes. J Cell Sci 104:261–274
Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF (1996) Abnormal centrosome amplification in the absence of p53. Science 271:1744–1747
Balczon R (1996) The centrosome in animal cells and its functional homologs in plant and yeast cells. Int Rev Cytol 169:25–82
Doxsey S (2001) Re-evaluating centrosome function. Nat Rev Mol Cell Biol 2:688–698
Bornens M (2002) Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol 14:25–34
Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M (1996) Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci 109:3089–3102
Moudjou M, Bordes N, Paintrand M, Bornens M (1996) γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci 109:875–887
Baas PW, Joshi HC (1992) Gamma-tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J Cell Biol 119:171–178
Joshi HC, Palacios MJ, McNamara L, Cleveland DW (1992) γ-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature 356:80–83
Oakley CE, Oakley BR (1989) Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338:662–664
Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA (1999) Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol 147:321–334
Askham JM, Vaughan KT, Goodson HV, Morrison EE (2002) Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell 13:3627–3645
Quintyne NJ, Schroer TA (2002) Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J Cell Biol 159:245–254
Louie RK, Bahmanyar S, Siemers KA, Votin V, Chang P, Stearns T, Nelson WJ, Barth AI (2004) Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J Cell Sci 117:1117–1128
Delgehyr N, Sillibourne J, Bornens M (2005) Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci 118:1565–1575
Moisoi N, Erent M, Whyte S, Martin S, Bayley PM (2002) Calmodulin-containing substructures of the centrosomal matrix released by microtubule perturbation. J Cell Sci 115:2367–2379
Dodson H, Wheatley SP, Morrison CG (2007) Involvement of centrosome amplification in radiation-induced mitotic catastrophe. Cell Cycle 63:364–370
Yih LH, Tseng YY, Wu YC, Lee TC (2006) Induction of centrosome amplification during arsenite-induced mitotic arrest in CGL-2 cells. Cancer Res 66:2098–2106
Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S (2007) Loss of centrosome integrity induces p38-p53-p21-dependent G1–S arrest. Nat Cell Biol 9:160–170
Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL, Tanaka M (2000) A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 19:5281–5290
Kanthou C, Greco O, Stratford A, Cook I, Knight R, Benzakour O, Tozer G (2004) The tubulin-binding agent combretastatin A-4-phosphate arrests endothelial cells in mitosis and induces mitotic cell death. Am J Pathol 165:1401–1411
Vitale I, Antoccia A, Cenciarelli C, Crateri P, Meschini S, Arancia G, Pisano C, Tanzarella C (2007) Combretastatin CA-4 and combretastatin derivative induce mitotic catastrophe-dependent on spindle checkpoint and caspase-3 activation in non-small cell lung cancer cells. Apoptosis 12:155–166
Nabha SM, Mohammad RM, Dandashi MH, Coupaye-Gerard B, Aboukameel A, Pettit GR, Al-Katib AM (2002) Combretastatin-A4 prodrug induces mitotic catastrophe in chronic lymphocytic leukemia cell line independent of caspase activation and poly(ADP-ribose) polymerase cleavage. Clin Cancer Res 8:2735–2741
Castedo M, Perfettini JL, Roumier T, Yakushijin K, Horne D, Medema R, Kroemer G (2004) The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene 23:4353–4361
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837
Roninson IB, Broude EV, Chang BD (2007) If not apoptosis then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat 4:303–313
Ianzini F, Mackey MA (1997) Spontaneous premature chromosome condensation and mitotic catastrophe following irradiation of HeLa S3 cells. Int J Radiat Biol 72:409–421
Vitale I, Antoccia A, Crateri P, Leone S, Arancia G, Tanzarella C (2005) Caspase-independent apoptosis is activated by diazepam-induced mitotic failure in HeLa cells, but not in human primary fibroblasts. Apoptosis 10:909–920
Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N (1992) Incresased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated protein MAP1B, MAP2 or tau. J Cell Sci 103:953–964
Sato C, Kuriyama R, Nishizawa K (1983) Microtubule-organizing centers abnormal in number, structure, and nucleating activity in X-irradiated mammalian cells. J Cell Biol 96:776–782
Ochi T, Nakajima F, Nasui M (1999) Distribution of gamma-tubulin in multipolar spindles and multinucleated cells induced by dimethylarsinic acid, a methylated derivative of inorganic arsenics, in Chinese hamster V79 cells. Toxicology 136:79–88
Ochi T (2000) Induction of centrosome injury, multipolar spindles and multipolar division in cultured V79 cells exposed to dimethylarsinic acid: role for microtubules in centrosome dynamics. Mutat Res 454:21–33
Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ (1998) Pericentrin and gamma-tubulin form a protein complex into a novel lattice at the centrosome. J Cell Biol 141:163–174
Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M (2000) Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci 113:3013–3023
Stillwell EE, Zhou J, Joshi HC (2004) Human ninein is a centrosomal autoantigen recognized by CREST patient sera and plays a regulatory role in microtubule nucleation. Cell Cycle 3:923–930
Mahoney M, Goshima G, Douglass AD, Vale RD (2006) Making microtubules and mitotic spindle in cells without functional centrosomes. Curr Biol 16:564–569
Khodjakov A, Copenagle L, Gordon B, Compton DA, Kapoor TM (2003) Minus-end capture of preformed kinetochore fibers contribute to spindle morphogenesis. J Cell Biol 160:671–683
Janson ME, Setty TG, Paoletti A, Tran PT (2005) Efficient formation of bipolar microtubule bundles requires microtubule-bound gamma-tubulin complexes. J Cell Biol 169:297–308
Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M (2005) Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol 7:961–968
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Yamaguchi H, Chen J, Bhalla K, Wang HG (2004) Regulation of bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem 279:39431–39437
Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ (2004) Mitosis-specific anchoring of gamma-tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell 15:3642–3657
Moss DK, Betin VM, Malesinski SD, Lane JD (2006) A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci 119:2362–2374
Moss DK, Lane JD (2006) Microtubules: forgotten players in the apoptotic execution phase. Trends Cell Biol 16:330–338
Acknowledgments
Chiara Cenciarelli is a PhD fellow at the Department of Biology, Università Roma Tre. This work was partly financed by SIGMA-TAU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cenciarelli, C., Tanzarella, C., Vitale, I. et al. The tubulin-depolymerising agent combretastatin-4 induces ectopic aster assembly and mitotic catastrophe in lung cancer cells H460. Apoptosis 13, 659–669 (2008). https://doi.org/10.1007/s10495-008-0200-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0200-2