Abstract
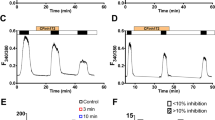
The conductance of oocytes expressing T338C CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) exhibits variable responses to dithiothreitol (DTT) and 2-mercaptoethanol (2-ME) that we proposed might be due to the extraction of copper from an adventitious binding site (Liu et al. J Biol Chem 281(12):8275–8285, 2006). In order to study the origins of variability in chemical reactivity of T338C CFTR channels, oocytes expressing T338C CFTR were exposed to BCNU (bischloroethylnitrosourea), an inhibitor of glutathione reductase. BCNU treatment caused a significant reduction of initial conductance and an increase in the response to 2-ME or DTT, suggesting a direct or indirect influence of intracellular glutathione (GSH), a major determinant of the disposition of intracellular copper. Single-channel recordings indicated that T338C CFTR channels not exposed to 2-ME or DTT exhibited multiple conductance levels not seen in T338A CFTR channels. Exposure to BCNU shifted the distribution of single-channel current amplitudes towards lower values, whereas exposure to DTT favored higher amplitudes. These results suggest that the altered chemical state of T338C channels is associated with a decreased single-channel conductance and that intracellular factors (most likely GSH) may modulate the propensity of the channel to form these altered states.






Similar content being viewed by others
References
Ascone I, Longo A et al (1993) An X-ray absorption study of the reconstitution process of bovine Cu,Zn superoxide dismutase by Cu(I)-glutathione complex. FEBS Lett 322(2):165–167
Ballatori N, Wang W et al (1996) An endogenous ATP-sensitive glutathione S-conjugate efflux mechanism in Xenopus laevis oocytes. Am J Physiol 270(5 Pt 2):R1156–R1162
Becq F, Jensen TJ et al (1994) Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proc Natl Acad Sci USA 91(19):9160–9164
Bethin KE, Cimato TR et al (1995a) Copper binding to mouse liver S-adenosylhomocysteine hydrolase and the effects of copper on its levels. J Biol Chem 270(35):20703–20711
Bethin KE, Petrovic N et al (1995b) Identification of a major hepatic copper binding protein as S-adenosylhomocysteine hydrolase. J Biol Chem 270(35):20698–20702
Changela A, Chen K et al (2003) Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301(5638):1383–1387
Ciriolo MR, Desideri A et al (1990) Reconstitution of Cu,Zn-superoxide dismutase by the Cu(I).glutathione complex. J Biol Chem 265(19):11030–11034
Eklow L, Moldeus P et al (1984) Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem 138(3):459–463
Ferreira AM, Ciriolo MR et al (1993) Copper(I) transfer into metallothionein mediated by glutathione. Biochem J 292(Pt 3):673–676
Finney LA, O’Halloran TV (2003) Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300(5621):931–936
Freedman JH, Ciriolo MR et al (1989) The role of glutathione in copper metabolism and toxicity. J Biol Chem 264(10):5598–5605
Heidemann SR, Hamborg MA (1984) The thiol-disulfide balance during maturation of Xenopus laevis oocytes. J Exp Zool 231(1):93–100
Kogan I, Ramjeesingh M et al (2003) CFTR directly mediates nucleotide-regulated glutathione flux. Embo J 22(9):1981–1989
Krezel A, Lesniak W et al (2001) Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant. J Inorg Biochem 84(1–2):77–88
Linsdell P, Hanrahan JW (1998) Glutathione permeability of CFTR. Am J Physiol 275(1 Pt 1):C323–C326
Linsdell P, Zheng SX et al (1998) Non-pore lining amino acid side chains influence anion selectivity of the human CFTR Cl- channel expressed in mammalian cell lines. J Physiol 512(Pt 1):1–16
Liu X, Alexander C et al (2006) Variable reactivity of an engineered cysteine at position 338 in cystic fibrosis transmembrane conductance regulator reflects different chemical states of the thiol. J Biol Chem 281(12):8275–8285
Liu X, Zhang ZR et al (2004) CFTR: A Cysteine at Position 338 in TM6 Senses a Positive Electrostatic Potential in the Pore. Biophys J 87(6):3826–3241
Luk E, Jensen LT et al (2003) The many highways for intracellular trafficking of metals. J Biol Inorg Chem 8(8):803–809. Epub 2003 Sep 27
Meredith MJ, Reed DJ (1983) Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem Pharmacol 32(8):1383–1388
Messana JM, Cieslinski DA et al (1988) Glutathione protects against exogenous oxidant injury to rabbit renal proximal tubules. Am J Physiol 255(5 Pt 2):F874–F884
Nomizu T, Falchuk KH et al (1993) Zinc, iron, and copper contents of Xenopus laevis oocytes and embryos. Mol Reprod Dev 36(4):419–423
O’Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275(33):25057–25060
Ohta Y, Shiraishi N et al (2001) Ascorbate-induced high-affinity binding of copper to cytosolic proteins. Biochem Biophys Res Commun 287(4):888–894
Palumaa P, Kangur L et al (2004) Metal-binding mechanism of Cox17, a copper chaperone for cytochrome c oxidase. Biochem J 382(Pt 1):307–314
Rabenstein DL (1989) Metal complexes of glutathione and their biological significance. In: Dolphin D, Poulson R, Avramovic O (eds) Glutathione: chemical, biochemical and medical aspects. Wiley-Interscience, York, Part A, pp 147–186
Rae TD, Schmidt PJ et al (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284(5415):805–808
Romero FJ, Zukowski D et al (1997) Glutathione content of V79 cells in two- or three-dimensional culture. Am J Physiol 272(5 Pt 1):C1507–C1512
Shinohara K, Tanaka KR (1979) Mechanism of inhibition of red blood cell glutathione reductase activity by BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea). Clin Chim Acta 92(2):147–152
Sivaraja V, Kumar TK et al (2006) Copper binding affinity of S100A13, a key component of the FGF-1 nonclassical copper-dependent release complex. Biophys J 91(5):1832–1843 Epub 2006 Jun 9
Smith SS, Liu X et al (2001) CFTR Covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J Gen Physiol 118(4):407–432
Yang L, McRae R et al (2005) Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron X-ray fluorescence microscopy. Proc Natl Acad Sci USA 102(32):11179–11184
Zhang Z, Cui G et al (2005) Determination of the functional unit of the cystic fibrosis transmembrane conductance regulator chloride channel. One polypeptide forms one pore. J Biol Chem 280(1):458–468
Acknowledgements
I am indebted to Dr. David Dawson for his mentoring, financial support and review of this manuscript. I also thank the members of the Dawson lab for their support. Dr. Michael J. Meredith’s discussion of BCNU is greatly appreciated. This work was supported by the National Institute for Diabetes, Digestive and Kidney Diseases (NIH DK45880 to Dawson). During a portion of this work the author was supported by the National Institute for Diabetes, Digestive and Kidney Diseases (DK60312) and (DK070755).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X. A possible role for intracellular GSH in spontaneous reaction of a cysteine (T338C) engineered into the Cystic Fibrosis Transmembrane Conductance Regulator. Biometals 21, 277–287 (2008). https://doi.org/10.1007/s10534-007-9117-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-007-9117-4