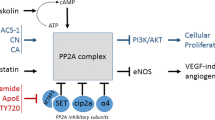
Abstract
Protein phosphatase-2A (PP2A) is one of the major cellular serine-threonine phosphatases and is involved in the regulation of cell homeostasis through the negative regulation of signaling pathways initiated by protein kinases. As several cancers are characterized by the aberrant activity of oncogenic kinases, it was not surprising that a phosphatase like PP2A has progressively been considered as a potential tumor suppressor. Indeed, multiple solid tumors (e.g. melanomas, colorectal carcinomas, lung and breast cancers) present with genetic and/or functional inactivation of different PP2A subunits and, therefore, loss of PP2A phosphatase activity towards certain substrates. Likewise, impaired PP2A phosphatase activity has been linked to B-cell chronic lymphocytic leukemia, Philadelphia-chromosome positive acute lymphoblastic leukemia and blast crisis chronic myelogenous leukemia. Remarkably, drugs such as forskolin, 1,9-dideoxy-forskolin and FTY720 which lead to PP2A activation effectively antagonize leukemogenesis in both in vitro and in vivo models of these cancers. Thus, PP2A is now in the spotlight as a highly promising drugable target for the development of a new series of anticancer agents potentially capable of overcoming drug-resistance induced in patients by continuous exposure to kinase inhibitor monotherapy. Herein, we review current knowledge of PP2A biology and function with particular emphasis on its tumor suppressor activity and possible therapeutic implications in cancer.


Similar content being viewed by others
Abbreviations
- ALL:
-
acute lymphoblastic leukemia
- CML:
-
chronic myelogenous leukemia
- hnRNP:
-
heterogeneous nuclear ribonucleoprotein
- MAPK:
-
mitogen activated protein kinase
- Ph:
-
philadelphia chromosome
- PP2A:
-
protein phosphatase 2A
References
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100(1), 57–70.
Todd, R., & Wong, D. T. (1999). Oncogenes. Anticancer Research, 19(6A), 4729–4746.
Sherr, C. J. (2004). Principles of tumor suppression. Cell, 116(2), 235–246.
Yokota, J. (2000). Tumor progression and metastasis. Carcinogenesis, 21(3), 497–503.
Hunter, T. (1995). Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell, 80(2), 225–236.
Levine, A. J., & Broach, J. R. (1995). Oncogenes and cell proliferation. Current Opinion in Genetics & Development, 5(1), 1–4.
Calabretta, B., & Perrotti, D. (2004). The biology of CML blast crisis. Blood, 103(11), 4010–4022.
Westbrook, C. A., Hooberman, A. L., Spino, C., Dodge, R. K., Larson, R. A., Davey, F., et al. (1992). Clinical significance of the BCR-ABL fusion gene in adult acute lymphoblastic leukemia: A Cancer and Leukemia Group B Study (8762). Blood, 80(12), 2983–2990.
Tonks, N. K. (2006). Protein tyrosine phosphatases: From genes, to function, to disease. Nature Reviews Molecular Cell Biology, 7(11), 833–846.
Janssens, V., Goris, J., & Van Hoof, C. (2005). PP2A: the expected tumor suppressor. Current Opinion in Genetics & Development, 15(1), 34–41.
Perrotti, D., & Neviani, P. (2006). ReSETting PP2A tumour suppressor activity in blast crisis and imatinib-resistant chronic myelogenous leukaemia. British Journal of Cancer, 95(7), 775–781.
Janssens, V., & Goris, J. (2001). Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochemical Journal, 353(Pt 3), 417–439.
Schonthal, A. H. (2001). Role of serine/threonine protein phosphatase 2A in cancer. Cancer Letter, 170(1), 1–13.
Lechward, K., Awotunde, O. S., Swiatek, W., & Muszynska, G. (2001). Protein phosphatase 2A: Variety of forms and diversity of functions. Acta Biochimica Polonica, 48(4), 921–933.
Healy, A. M., Zolnierowicz, S., Stapleton, A. E., Goebl, M., DePaoli-Roach, A. A., & Pringle, J. R. (1991). CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: Identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Molecular and Cellular Biology, 11(11), 5767–5780.
Mayer-Jaekel, R. E., Ohkura, H., Gomes, R., Sunkel, C. E., Baumgartner, S., Hemmings, B. A., et al. (1993). The 55 kd regulatory subunit of Drosophila protein phosphatase 2A is required for anaphase. Cell, 72(4), 621–633.
Schild, A., Schmidt, K., Lim, Y. A., Ke, Y., Ittner, L. M., Hemmings, B. A., et al. (2006). Altered levels of PP2A regulatory B/PR55 isoforms indicate role in neuronal differentiation. International Journal of Developmental Neuroscience, 24(7), 437–443.
Okamoto, K., Li, H., Jensen, M. R., Zhang, T., Taya, Y., Thorgeirsson, S. S., et al. (2002). Cyclin G recruits PP2A to dephosphorylate Mdm2. Molecular Cell, 9(4), 761–771.
Voorhoeve, P. M., Hijmans, E. M., & Bernards, R. (1999). Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59, and the retinoblastoma-related p107 protein. Oncogene, 18(2), 515–524.
Sontag, E. (2001). Protein phosphatase 2A: The Trojan Horse of cellular signaling. Cell Signal, 13(1), 7–16.
Sontag, E., Fedorov, S., Kamibayashi, C., Robbins, D., Cobb, M., & Mumby, M. (1993). The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell, 75(5), 887–897.
Lee, T. H., Turck, C., & Kirschner, M. W. (1994). Inhibition of cdc2 activation by INH/PP2A. Molecular Biology of the Cell, 5(3), 323–338.
Van Hoof, C., & Goris, J. (2003). Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochimica et Biophysica Acta, 1640(2–3), 97–104.
Yang, J., Fan, G. H., Wadzinski, B. E., Sakurai, H., & Richmond, A. (2001). Protein phosphatase 2A interacts with and directly dephosphorylates RelA. Journal of Biological Chemistry, 276(51), 47828–47833.
Ivaska, J., Nissinen, L., Immonen, N., Eriksson, J. E., Kahari, V. M., & Heino, J. (2002). Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Molecular and Cellular Biology, 22(5), 1352–1359.
Peterson, R. T., Desai, B. N., Hardwick, J. S., & Schreiber, S. L. (1999). Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4438–4442.
Montagne, J., Stewart, M. J., Stocker, H., Hafen, E., Kozma, S. C., & Thomas, G. (1999). Drosophila S6 kinase: a regulator of cell size. Science, 285(5436), 2126–2129.
Hornstein, E., Tang, H., & Meyuhas, O. (2001). Mitogenic and nutritional signals are transduced into translational efficiency of TOP mRNAs. Cold Spring Harbor Symposia on Quantitative Biology, 66, 477–484.
Li, X., Yost, H. J., Virshup, D. M., & Seeling, J. M. (2001). Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. European Molecular Biology Organization Journal, 20(15), 4122–4131.
Sontag, J. M., & Sontag, E. (2006). Regulation of cell adhesion by PP2A and SV40 small tumor antigen: An important link to cell transformation. Cellular and Molecular Life Sciences, 63(24), 2979–2991.
Xu, Y., Xing, Y., Chen, Y., Chao, Y., Lin, Z., Fan, E., et al. (2006). Structure of the protein phosphatase 2A holoenzyme. Cell, 127(6), 1239–1251.
Longin, S., Zwaenepoel, K., Louis, J. V., Dilworth, S., Goris, J., & Janssens, V. (2007). Selection of protein phosphatase 2A regulatory subunits is mediated by the C-terminus of the catalytic subunit. Journal of Biological Chemistry, 282, 26971–26980.
Li, M., & Damuni, Z. (1998). I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. Methods in Molecular Biology, 93, 59–66.
Kovacech, B., Kontsekova, E., Zilka, N., Novak, P., Skrabana, R., Filipcik, P., et al. (2007). A novel monoclonal antibody DC63 reveals that inhibitor 1 of protein phosphatase 2A is preferentially nuclearly localised in human brain. Federation of European Biochemical Societies Letters, 581(4), 617–622.
Li, M., Makkinje, A., & Damuni, Z. (1996). The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. Journal of Biological Chemistry, 271(19), 11059–11062.
Adachi, Y., Pavlakis, G. N., & Copeland, T. D. (1994). Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. Journal of Biological Chemistry, 269(3), 2258–2262.
Adler, H. T., Nallaseth, F. S., Walter, G., & Tkachuk, D. C. (1997). HRX leukemic fusion proteins form a heterocomplex with the leukemia-associated protein SET and protein phosphatase 2A. Journal of Biological Chemistry, 272(45), 28407–28414.
Neviani, P., Santhanam, R., Trotta, R., Notari, M., Blaser, B. W., Liu, S., et al. (2005). The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell, 8(5), 355–368.
Neviani, P., Santhanam, R., Oaks, J. J., Eiring, A. M., Notari, M., Blaser, B. W., et al. (2007). FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. Journal of Clinical Investigation, 117(9), 2408–2421.
Trotta, R., Ciarlariello, D., Col, J. D., Allard 2nd, J., Neviani, P., Santhanam, R., et al. (2007). The PP2A inhibitor SET regulates natural killer cell IFN-{gamma} production. Journal of Experimental Medicine, 204, 2397–2405.
Bialojan, C., & Takai, A. (1988). Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochemical Journal, 256(1), 283–290.
Arroyo, J. D., & Hahn, W. C. (2005). Involvement of PP2A in viral and cellular transformation. Oncogene, 24(52), 7746–7755.
Junttila, M. R., Puustinen, P., Niemela, M., Ahola, R., Arnold, H., Bottzauw, T., et al. (2007). CIP2A inhibits PP2A in human malignancies. Cell, 130(1), 51–62.
Sablina, A. A., Chen, W., Arroyo, J. D., Corral, L., Hector, M., Bulmer, S. E., et al. (2007). The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell, 129(5), 969–982.
Francia, G., Poulsom, R., Hanby, A. M., Mitchell, S. D., Williams, G., McKee, P., et al. (1999). Identification by differential display of a protein phosphatase-2A regulatory subunit preferentially expressed in malignant melanoma cells. International Journal of Cancer, 82(5), 709–713.
Ito, A., Kataoka, T. R., Watanabe, M., Nishiyama, K., Mazaki, Y., Sabe, H., et al. (2000). A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. European Molecular Biology Organization Journal, 19(4), 562–571.
Chen, W., Possemato, R., Campbell, K. T., Plattner, C. A., Pallas, D. C., & Hahn, W. C. (2004). Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell, 5(2), 127–136.
Ito, A., Koma, Y., Watabe, K., Nagano, T., Endo, Y., Nojima, H., et al. (2003). A truncated isoform of the protein phosphatase 2A B56gamma regulatory subunit may promote genetic instability and cause tumor progression. American Journal of Pathology, 162(1), 81–91.
Silverstein, A. M., Barrow, C. A., Davis, A. J., & Mumby, M. C. (2002). Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4221–4226.
Li, X., Scuderi, A., Letsou, A., & Virshup, D. M. (2002). B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Molecular and Cellular Biology, 22(11), 3674–3684.
Calin, G. A., di Iasio, M. G., Caprini, E., Vorechovsky, I., Natali, P. G., Sozzi, G., et al. (2000). Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene, 19(9), 1191–1195.
Ruediger, R., Pham, H. T., & Walter, G. (2001). Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene, 20(15), 1892–1899.
Takagi, Y., Futamura, M., Yamaguchi, K., Aoki, S., Takahashi, T., & Saji, S. (2000). Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut, 47(2), 268–2671.
Wang, S. S., Esplin, E. D., Li, J. L., Huang, L., Gazdar, A., Minna, J., et al. (1998). Alterations of the PPP2R1B gene in human lung and colon cancer. Science, 282(5387), 284–287.
Esplin, E. D., Ramos, P., Martinez, B., Tomlinson, G. E., Mumby, M. C., & Evans, G. A. (2006). The glycine 90 to aspartate alteration in the Abeta subunit of PP2A (PPP2R1B) associates with breast cancer and causes a deficit in protein function. Genes Chromosomes Cancer, 45(2), 182–190.
Baysal, B. E., Willett-Brozick, J. E., Taschner, P. E., Dauwerse, J. G., Devilee, P., & Devlin, B. (2001). A high-resolution integrated map spanning the SDHD gene at 11q23: A 1.1-Mb BAC contig, a partial transcript map and 15 new repeat polymorphisms in a tumour-suppressor region. European Journal of Human Genetics, 9(2), 121–129.
Camonis, J. H., & White, M. A. (2005). Ral GTPases: corrupting the exocyst in cancer cells. Trends in Cell Biology, 15(6), 327–332.
Tamaki, M., Goi, T., Hirono, Y., Katayama, K., & Yamaguchi, A. (2004). PPP2R1B gene alterations inhibit interaction of PP2A-Abeta and PP2A-C proteins in colorectal cancers. Oncology Reports, 11(3), 655–659.
Chen, W., Arroyo, J. D., Timmons, J. C., Possemato, R., & Hahn, W. C. (2005). Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Research, 65(18), 8183–8192.
Trotman, L. C., Alimonti, A., Scaglioni, P. P., Koutcher, J. A., Cordon-Cardo, C., & Pandolfi, P. P. (2006). Identification of a tumour suppressor network opposing nuclear Akt function. Nature, 441(7092), 523–527.
Soo Hoo, L., Zhang, J. Y., & Chan, E. K. (2002). Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene, 21(32), 5006–5015.
Arnold, H. K., & Sears, R. C. (2006). Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Molecular and Cellular Biology, 26(7), 2832–2844.
Sears, R. C. (2004). The life cycle of C-myc: from synthesis to degradation. Cell Cycle, 3(9), 1133–1137.
Yeh, E., Cunningham, M., Arnold, H., Chasse, D., Monteith, T., Ivaldi, G., et al. (2004). A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nature Cell Biology, 6(4), 308–318.
Escamilla-Powers, J. R., & Sears, R. C. (2007). A conserved pathway that controls c-Myc protein stability through opposing phosphorylation events occurs in yeast. Journal of Biological Chemistry, 282(8), 5432–5442.
Kalla, C., Scheuermann, M. O., Kube, I., Schlotter, M., Mertens, D., Dohner, H., et al. (2007). Analysis of 11q22-q23 deletion target genes in B-cell chronic lymphocytic leukaemia: evidence for a pathogenic role of NPAT, CUL5, and PPP2R1B. European Journal of Cancer, 43(8), 1328–1335.
Dohner, H., Stilgenbauer, S., James, M. R., Benner, A., Weilguni, T., Bentz, M., et al. (1997). 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood, 89(7), 2516–2522.
Zhu, Y., Loukola, A., Monni, O., Kuokkanen, K., Franssila, K., Elonen, E., et al. (2001). PPP2R1B gene in chronic lymphocytic leukemias and mantle cell lymphomas. Leukaemia and Lymphoma, 41(1–2), 177–183.
Liu, Q., Zhao, X., Frissora, F., Ma, Y., Santhanam, R., Jarjoura, D., et al. (2008). FTY720 demonstrates promising pre-clinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood, 111, 275–284.
Dumont, F. J. (2005). Fingolimod. Mitsubishi Pharma/Novartis. IDrugs, 8(3), 236–253.
Virley, D. J. (2005). Developing therapeutics for the treatment of multiple sclerosis. NeuroRx, 2(4), 638–649.
Budde, K., Schmouder, R. L., Brunkhorst, R., Nashan, B., Lucker, P. W., Mayer, T., et al. (2002). First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. Journal of the American Society of Nephrology, 13(4), 1073–1083.
Skerjanec, A., Tedesco, H., Neumayer, H. H., Cole, E., Budde, K., Hsu, C. H., et al. (2005). FTY720, a novel immunomodulator in de novo kidney transplant patients: Pharmacokinetics and exposure–response relationship. Journal of Clinical Pharmacology, 45(11), 1268–1278.
Brinkmann, V. (2004). FTY720: mechanism of action and potential benefit in organ transplantation. Yonsei Medical Journal, 45(6), 991–997.
Kovarik, J. M., Schmouder, R., Barilla, D., Riviere, G. J., Wang, Y., & Hunt, T. (2004). Multiple-dose FTY720: Tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. Journal of Clinical Pharmacology, 44(5), 532–537.
Iervolino, A., Santilli, G., Trotta, R., Guerzoni, C., Cesi, V., Bergamaschi, A., et al. (2002). hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis. Molecular and Cellular Biology, 22(7), 2255–2266.
Perrotti, D., & Neviani, P. (2007). From mRNA metabolism to cancer therapy: Chronic myelogenous leukemia shows the way. Clinical Cancer Research, 13(6), 1638–1642.
Sato, S., Fujita, N., & Tsuruo, T. (2000). Modulation of Akt kinase activity by binding to Hsp90. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 10832–10837.
Yokoyama, N., Reich, N. C., & Miller, W. T. (2001). Involvement of protein phosphatase 2A in the interleukin-3-stimulated Jak2-Stat5 signaling pathway. Journal of Interferon and Cytokine Research, 21(6), 369–378.
Chiang, C. W., Kanies, C., Kim, K. W., Fang, W. B., Parkhurst, C., Xie, M., et al. (2003). Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Molecular and Cellular Biology, 23(18), 6350–6362.
Gomez, N., & Cohen, P. (1991). Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature, 353(6340), 170–173.
Avni, D., Yang, H., Martelli, F., Hofmann, F., ElShamy, W. M., Ganesan, S., et al. (2003). Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Molecular Cell, 12(3), 735–746.
Cortez, D., Reuther, G., & Pendergast, A. M. (1997). The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene, 15(19), 2333–2342.
Feschenko, M. S., Stevenson, E., Nairn, A. C., & Sweadner, K. J. (2002). A novel cAMP-stimulated pathway in protein phosphatase 2A activation. Journal of Pharmacology and Experimental Therapeutics, 302(1), 111–118.
Matsuoka, Y., Nagahara, Y., Ikekita, M., & Shinomiya, T. (2003). A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. British Journal of Pharmacology, 138(7), 1303–1312.
Liedtke, M., Pandey, P., Kumar, S., Kharbanda, S., & Kufe, D. (1998). Regulation of Bcr-Abl-induced SAP kinase activity and transformation by the SHPTP1 protein tyrosine phosphatase. Oncogene, 17(15), 1889–1892.
Lim, Y. M., Wong, S., Lau, G., Witte, O. N., & Colicelli, J. (2000). BCR/ABL inhibition by an escort/phosphatase fusion protein. Proceedings of the National Academy of Sciences of the United States of America, 97(22), 12233–12238.
Chen, P., Levis, M., Brown, P., Kim, K. T., Allebach, J., & Small, D. (2005). FLT3/ITD mutation signaling includes suppression of SHP-1. Journal of Biological Chemistry, 280(7), 5361–5369.
Wu, C., Guan, Q., Wang, Y., Zhao, Z. J., & Zhou, G. W. (2003). SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. Journal of Cellular Biochemistry, 90(5), 1026–1037.
Wu, C., Sun, M., Liu, L., & Zhou, G. W. (2003). The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene, 306, 1–12.
Ding, X., & Staudinger, J. L. (2005). Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. Journal of Pharmacology and Experimental Therapeutics, 312(2), 849–856.
Seamon, K., & Daly, J. W. (1981). Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. Journal of Biological Chemistry, 256(19), 9799–9801.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute CA095512 (D.P.), NIH, Bethesda, MD; the US Army, Chronic Myelogenous Leukemia Research Program, DAMD17-03-1-0184 and W81XWH-07-1-0270 (D.P) and by the American-Italian Cancer Foundation (P.N). D.P. is a Scholar of the Leukemia and Lymphoma Society. The use of PP2A activating drugs for the treatment of Ph1 leukemias is currently patent pending: Perrotti D et al. (PCT/US2006/10882) “Activation of PP2A in the Treatment of Ph1 Leukemias” Application Serial Number: 60/665,091 final application pending.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perrotti, D., Neviani, P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Rev 27, 159–168 (2008). https://doi.org/10.1007/s10555-008-9119-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9119-x