Abstract
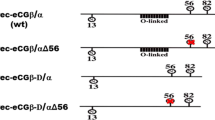
The strategy of translationally fusing the subunits of heterodimeric proteins into single chain molecules is often used to overcome the mutagenesis-induced defects in subunit interactions. The approach of fusing the α and β subunits of human Chorionic Gonadotropin (hCG) to produce a single chain hormone (phCGαβ) was used to investigate roles of critical residues of the α subunit in hormone receptor interaction and biological activity. The α subunit was mutated using PCR-based site-directed mutagenesis, fused to the wild type β subunit and the fusion protein was expressed using Pichia pastoris expression system. Following partial purification, the mutant proteins were extensively characterized using immunological probes, receptor assays, and in vitro bioassays. The mutation hCGα P38A, which disrupts subunit interaction in the heterodimeric molecule, produced a fusion molecule exhibiting altered subunit interactions as judged by the immunological criteria, but could bind to the receptor with lower affinity and elicit biological response. Mutation of hCGα T54A disrupting the glycosylation at Asparagine 52, believed to be important for bioactivity, also yielded a biologically active molecule suggesting that the glycosylation at this site is not as critical for bioactivity as it is in the case of the heterodimer. The fusion protein approach was also used to generate a superagonist of hormone action. Introduction of four lysine residues in the Loop 1 of the α subunit led to the generation of a mutant having higher affinity for the receptor and enhanced bioactivity. Immunological characterization of single chain molecules revealed that the interactions between the subunits were not identical to those seen in the heterodimeric hormone, and the subunits appeared to retain their isolated conformations, and also retained the ability to bind to the receptors and elicit response. These data suggest the plasticity of the hormone-receptor interactions.






Similar content being viewed by others
References
Pierce, J.G., Parsons, T.F.: Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50, 465–495 (1981)
Wu, H., Lustbader, J.W., Liu, Y., Canfield, R.E., Hendrickson, W.A.: Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure 2, 545–558 (1994)
Lapthorn, A.J., Harris, D.C., Littlejohn, A., Lustbader, J.W., Canfield, R.E., Machin, K.J., Morgan, F.J., Isaacs, N.W.: Crystal structure of human chorionic gonadotropin. Nature 369, 455–461 (1994)
Fox, K.M., Dias, J.A., Van Roey, P.: Three-dimensional structure of human follicle-stimulating hormone. Mol. Endocrinol. 15, 378–389 (2001)
Sugahara, T., Grootenhuis, P.D., Sato, A., Kudo, M., Ben-Menahem, D., Pixley, M.R., Hsueh, A.J., Boime, I.: Expression of biologically active fusion genes encoding the common alpha subunit and either the CG beta or FSH beta subunits: role of a linker sequence. Mol. Cell. Endocrinol. 125, 71–77 (1996)
Narayan, P., Gray, J., Puett, D.: A biologically active single chain human chorionic gonadotropin analog with altered receptor binding properties. Endocrinology 141, 67–71 (2000)
Garcia-Campayo, V., Sato, A., Hirsch, B., Sugahara, T., Muyan, M., Hsueh, A.J., Boime, I.: Design of stable biologically active recombinant lutropin analogs. Nat. Biotechnol. 15, 663–667 (1997)
Sugahara, T., Pixley, M.R., Minami, S., Perlas, E., Ben-Menahem, D., Hsueh, A.J., Boime, I.: Biosynthesis of a biologically active single peptide chain containing the human common alpha and chorionic gonadotropin beta subunits in tandem. Proc. Natl. Acad. Sci. U. S. A. 92, 2041–2045 (1995)
Grossmann, M., Wong, R., Szkudlinski, M.W., Weintraub, B.D.: Human thyroid-stimulating hormone (hTSH) subunit gene fusion produces hTSH with increased stability and serum half-life and compensates for mutagenesis-induced defects in subunit association. J. Biol. Chem. 272, 21312–21316 (1997)
Sen Gupta, C., Dighe, R.R.: Biological activity of single chain chorionic gonadotropin, hCGalphabeta, is decreased upon deletion of five carboxyl terminal amino acids of the alpha subunit without affecting its receptor binding. J. Mol. Endocrinol. 24, 157–164 (2000)
Dighe, R.R., Murthy, G.S., Kurkalli, B.S., Moudgal, N.R.: Conformation of the alpha-subunit of glycoprotein hormones: a study using polyclonal and monoclonal antibodies. Mol. Cell. Endocrinol. 72, 63–70 (1990)
Dighe, R.R., Murthy, G.S., Moudgal, N.R.: Two simple and rapid methods to detect monoclonal antibodies with identical epitope specificities in a large population of monoclonal antibodies. J. Immunol. Methods 131, 229–236 (1990)
Higuchi, R., Krummel, B., Saiki, R.K.: A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16, 7351–7367 (1988)
Perrin, S., Gilliland, G.: Site-specific mutagenesis using asymmetric polymerase chain reaction and a single mutant primer. Nucleic Acids Res. 18, 7433–7438 (1990)
Sen Gupta, C., Dighe, R.R.: Hyperexpression of biologically active human chorionic gonadotropin using the methylotropic yeast, Pichia pastoris. J. Mol. Endocrinol. 22, 273–283 (1999)
Gadkari, R., Deshpande, R., Dighe, R.R.: Hyperexpression and purification of biologically active human luteinizing hormone and human chorionic gonadotropin using the methylotropic yeast, Pichia pastoris. Protein Expr. Purif. 32, 175–184 (2003)
Dighe, R.R., Moudgal, N.R.: Use of alpha- and beta-subunit specific antibodies in studying interaction of hCG with Leydig cell receptors. Arch. Biochem. Biophys. 225, 490–499 (1983)
Samaddar, M., Catterall, J.F., Dighe, R.R.: Expression of biologically active beta subunit of bovine follicle-stimulating hormone in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 10, 345–355 (1997)
Xia, H., Chen, F., Puett, D.: A region in the human glycoprotein hormone alpha-subunit important in holoprotein formation and receptor binding. Endocrinology 134, 1768–1770 (1994)
Grossmann, M., Szkudlinski, M.W., Dias, J.A., Xia, H., Wong, R., Puett, D., Weintraub, B.D.: Site-directed mutagenesis of amino acids 33–44 of the common alpha-subunit reveals different structural requirements for heterodimer expression among the glycoprotein hormones and suggests that cyclic adenosine 3′,5′-monophosphate production and growth promotion are potentially dissociable functions of human thyrotropin. Mol. Endocrinol. 10, 769–779 (1996)
Grossmann, M., Weintraub, B.D., Szkudlinski, M.W.: Novel insights into the molecular mechanisms of human thyrotropin action: structural, physiological, and therapeutic implications for the glycoprotein hormone family. Endocr. Rev. 18, 476–501 (1997)
De Beer, T., Van Zuylen, C.W., Leeflang, B.R., Hard, K., Boelens, R., Kaptein, R., Kamerling, J.P., Vliegenthart, J.F.: NMR studies of the free alpha subunit of human chorionic gonadotropin. Structural influences of N-glycosylation and the beta subunit on the conformation of the alpha subunit. Eur. J. Biochem. 241, 229–242 (1996)
Matzuk, M.M., Boime, I.: The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J. Cell. Biol. 106, 1049–1059 (1988)
Grossmann, M., Szkudlinski, M.W., Tropea, J.E., Bishop, L.A., Thotakura, N.R., Schofield, P.R., Weintraub, B.D.: Expression of human thyrotropin in cell lines with different glycosylation patterns combined with mutagenesis of specific glycosylation sites. Characterization of a novel role for the oligosaccharides in the in vitro and in vivo bioactivity. J. Biol. Chem. 270, 29378–29385 (1995)
Kikuchi, T., Koyama, M., Miyai, K., Kimura, T., Nishikiori, N., Azuma, C., Kusunoki, M., Saji, F., Tanizawa, O.: Loss of biological activity of human chorionic gonadotropin (hCG) by the amino acid substitution on the “CMGCC” region of the alpha-subunit. Mol. Cell. Endocrinol. 102, 1–7 (1994)
McFarland, K.C., Sprengel, R., Phillips, H.S., Kohler, M., Rosemblit, N., Nikolics, K., Segaloff, D.L., Seeburg, P.H.: Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245, 494–499 (1989)
Calvo, F.O., Ryan, R.J.: Inhibition of adenylyl cyclase activity in rat corpora luteal tissue by glycopeptides of human chorionic gonadotropin and the alpha-subunit of human chorionic gonadotropin. Biochemistry 24, 1953–1959 (1985)
Rebois, R.V., Liss, M.T.: Antibody binding to the beta-subunit of deglycosylated chorionic gonadotropin converts the antagonist to an agonist. J. Biol. Chem. 262, 3891–3896 (1987)
Heikoop, J.C., van den Boogaart, P., de Leeuw, R., Rose, U.M., Mulders, J.W., Grootenhuis, P.D.: Partially deglycosylated human choriogonadotropin, stabilized by intersubunit disulfide bonds, shows full bioactivity. Eur. J. Biochem. 253, 354–356 (1998)
Erbel, P.J., Haseley, S.R., Kamerling, J.P., Vliegenthart, J.F.: Studies on the relevance of the glycan at Asn-52 of the alpha-subunit of human chorionic gonadotropin in the alphabeta dimer. Biochem. J. 364, 485–495 (2002)
Szkudlinski, M.W., Teh, N.G., Grossmann, M., Tropea, J.E., Weintraub, B.D.: Engineering human glycoprotein hormone superactive analogues. Nat. Biotechnol. 14, 1257–1263 (1996)
Remy, J.J., Couture, L., Pantel, J., Haertle, T., Rabesona, H., Bozon, V., Pajot-Augy, E., Robert, P., Troalen, F., Salesse, R., Bidart, J.M.: Mapping of HCG-receptor complexes. Mol. Cell. Endocrinol. 125, 79–91 (1996)
Jiang, X., Dreano, M., Buckler, D.R., Cheng, S., Ythier, A., Wu, H., Hendrickson, W.A., el Tayar, N.: Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone–receptor interactions. Structure 3, 1341–1353 (1995)
Couture, L., Remy, J.J., Rabesona, H., Troalen, F., Pajot-Augy, E., Bozon, V., Haertle, T., Bidart, J.M., Salesse, R.: A defined epitope on the human choriogonadotropin alpha-subunit interacts with the second extracellular loop of the transmembrane domain of the lutropin/choriogonadotropin receptor. Eur. J. Biochem. 241, 627–632 (1996)
Sohn, J., Youn, H., Jeoung, M., Koo, Y., Yi, C., Ji, I., Ji, T.H.: Orientation of follicle-stimulating hormone (FSH) subunits complexed with the FSH receptor. Beta subunit toward the N terminus of exodomain and alpha subunit to exoloop 3. J. Biol. Chem. 278, 47868–47876 (2003)
Grossmann, M., Leitolf, H., Weintraub, B.D., Szkudlinski, M.W.: A rational design strategy for protein hormone superagonists. Nat. Biotechnol. 16, 871–875 (1998)
Leitolf, H., Tong, K.P., Grossmann, M., Weintraub, B.D., Szkudlinski, M.W.: Bioengineering of human thyrotropin superactive analogs by site-directed “lysine-scanning” mutagenesis. Cooperative effects between peripheral loops. J. Biol. Chem. 275, 27457–27465 (2000)
Jackson, A.M., Berger, P., Pixley, M., Klein, C., Hsueh, A.J., Boime, I.: The biological action of choriogonadotropin is not dependent on the complete native quaternary interactions between the subunits. Mol. Endocrinol. 13, 2175–2188 (1999)
Lobel, L., Pollak, S, Lustbader, B., Klein, J., Lustbader, J.W.: Bacterial expression of a natively folded extracellular domain fusion protein of the hFSH receptor in the cytoplasm of Escherichia coli. Protein Expr. Purif. 25, 124–133 (2002)
Ben-Menahem, D., Hyde, R., Pixley, M., Berger, P., Boime, I.: Synthesis of multi-subunit domain gonadotropin complexes: a model for alpha/beta heterodimer formation. Biochemistry 38, 15070–15077 (1999)
Kanda, M., Jablonka-Shariff, A., Sato, A., Pixley, M.R., Bos, E., Hiro’oka, T., Ben-Menahem, D., Boime, I.: Genetic fusion of an alpha-subunit gene to the follicle-stimulating hormone and chorionic gonadotropin-beta subunit genes: production of a bifunctional protein. Mol. Endocrinol. 13, 1873–1881 (1999)
Garcia-Campayo, V., Kumar, T.R., Boime, I.: Thyrotropin, follitropin, and chorionic gonadotropin expressed as a single multifunctional unit reveal remarkable permissiveness in receptor–ligand interactions. Endocrinology 143, 3773–3778 (2002)
Acknowledgments
The work presented here was supported by grants from the Department of Biotechnology, Government of India, the Indian Council of Medical Research, the University Grant Commission, New Delhi, and the Council for Scientific and Industrial Research, India (fellowship for SRS). We also wish to thank National Hormone Pituitary program, NIH, USA for providing the reference preparations of hormones.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Setlur, S.R., Dighe, R.R. Single chain human chorionic gonadotropin, hCGαβ: Effects of mutations in the α subunit on structure and bioactivity. Glycoconj J 24, 97–106 (2007). https://doi.org/10.1007/s10719-006-9016-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-006-9016-x