ABSTRACT
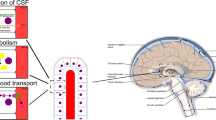
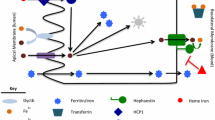
In the last decade, there has been substantial progress in understanding vectorial ligand transport through rodent and human choroid plexus (CP), the locus of the blood-CSF interface. In this Review, we enumerate the experimental data required to establish vectorial transport through CP and describe transporters involved in vectorial transport across CP. We also note how these transporters differ from those at the blood-brain barrier. The ligand (substrate) examples presented are methyltetrahydrofolate, methotrexate, leukotriene C4, nucleosides, thiamine monophosphate, prostaglandins, and digoxin. Our focus is on more definitive experiments, including animal and human transporter “knock-outs.” Finally, we discuss the neurochemical implications of vectorial transport through CP and the clinical implications of transporter polymorphisms and knockouts. Examples include descriptions of how vectorial transport through the CP for several micronutrients (e.g., methyltetrahydrofolate) nourishes the brain and how knowledge of CP vectorial transport can lead to important treatments.



Similar content being viewed by others
REFERENCES
Spector R, Johanson CE. The origin of deoxynucleosides in brain: implications for the study of neurogenesis and stem cell therapy. Pharm Res. 2007;24:859–67.
DiBenedetto FE, Bito LZ. Transport of prostaglandins and other eicosanoids by the choroid plexus: its characterization and physiological significance. J Neurochem. 1986;46:1725–31.
Spector R, Goetzl EJ. Role of concentrative leukotriene transport systems in the central nervous system. Biochem Pharmacol. 1986;35:2849–53.
Spector R, Lorenzo AV. The effects of salicylate and probenecid on the cerebrospinal fluid transport of penicillin, aminosalicyclic acid and iodide. J Pharmacol Exp Ther. 1974;188:55–65.
Bresler SE, Bresler VM, Kazbekov EN, Nikiforov AA, Vasilieva NN. On the active transport of organic acids (fluorescein) in the choroid plexus of the rabbit. Biochim Biophys Acta. 1979;550:110–9.
Spector R. Inhibition of methotrexate transport from cerebrospinal fluid by probenecid. Cancer Treat Rep. 1976;60:913–6.
Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1765–91.
Spector R, Johanson CE. Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J Neurochem. 2007;103:425–38.
Spector R. Nutrient transport systems in brain: 40 years of progress. J Neurochem. 2009;111:315–20.
Spector R. Riboflavin transport in the central nervous system. Characterization and effects of drugs. J Clin Invest. 1980;66:821–31.
Breen CM, Sykes DB, Fricker G, Miller DS. Confocal imaging of organic anion transport in intact rat choroid plexus. Am J Physiol Renal Physiol. 2002;282:F877–85.
Suleiman SA, Spector R. Purification and characterization of a folate binding protein from porcine choroid plexus. Arch Biochem Biophys. 1981;208:87–94.
Spector R. Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress. J Neurochem. 2010;112:13–23.
Johanson CE, Duncan 3rd JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10.
Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C et al. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol. 2008;510:497–507.
Eells JT, Spector R. Determination of ribonucleosides, deoxyribonucleosides, and purine and pyrimidine bases in adult rabbit cerebrospinal fluid and plasma. Neurochem Res. 1983;8:1307–20.
Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H et al. Analysis of methotrexate and folate transport by multidrug resistance associated protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62:3144–50.
Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance associated protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–7.
Gao B, Stieger B, Noe B, Fritschy JM, Meier PJ. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem. 1999;47:1255–64.
Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy JM, Meier PJ, Wolkoff AW. The choroid plexus epithelium is the site of the organic anion transport protein in the brain. Proc Natl Acad Sci USA. 1997;94:283–6.
Cattori V, van Montfoort JE, Stieger B, Landmann L, Meijer DK, Winterhalter KH et al. Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflugers Arch. 2001;443:188–95.
Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18.
Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science (New York, NY). 1995;268:866–9.
Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol. 2006;100:1392–9.
Alix E, Schmitt C, Strazielle N, Ghersi-Egea JF. Prostaglandin E2 metabolism in rat brain: Role of the blood-brain interfaces. Cerebrospinal Fluid Res. 2008;5:5.
Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M et al. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003;285:F1188–97.
Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos. 2003;31:1337–45.
Wijnholds J, deLange EC, Scheffer GL, van den Berg DJ, Mol CA, van der Valk M et al. Multidrug resistance associated protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest. 2000;105:279–85.
Lagas JS, Vlaming ML, Schinkel AH. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv. 2009;9:136–45.
Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–21.
Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J Biol Chem. 2001;276:2914–27.
Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43.
Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P et al. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85:354–63.
Spector R, Lorenzo AV. Folate transport by the choroid plexus in vitro. Science (New York, NY). 1975;187:540–2.
Mayatepek E, Lindner M, Zelezny R, Lindner W, Brandstetter G, Hoffmann GF. A severely affected infant with absence of cysteinyl leukotrienes in cerebrospinal fluid: further evidence that leukotriene C4-synthesis deficiency is a new neurometabolic disorder. Neuropediatrics. 1999;30:5–7.
Soderstrom M, Engblom D, Blomqvist A, Hammarstrom S. Expression of leukotriene C4 synthase mRNA by the choroid plexus in mouse brain suggests novel neurohormone functions of cysteinyl leukotrienes. Biochem Biophys Res Commun. 2003;307:987–90.
Karwatsky J, Leimanis M, Cai J, Gros P, Georges E. The leucotriene C4 binding sites in multidrug resistance associated protein 1 (ABCC1) include the first membrane multiple spanning domain. Biochemistry. 2005;44:340–51.
Spector R. Nucleoside transport in choroid plexus: mechanism and specificity. Arch Biochem Biophys. 1982;216:693–703.
Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M et al. The human multidrug resistance associated protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA. 2003;100:9244–9.
ACKNOWLEDGEMENTS
The authors wish to thank Michiko Spector for her aid in the preparation of the manuscript, and Julie Johanson and Arthur Messier for their assistance with graphics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spector, R., Johanson, C.E. Vectorial Ligand Transport Through Mammalian Choroid Plexus. Pharm Res 27, 2054–2062 (2010). https://doi.org/10.1007/s11095-010-0162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0162-2