Abstract
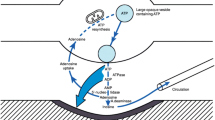
Extracellular nucleotides and nucleosides promote a vast range of physiological responses, via activation of cell surface purinergic receptors. Virtually all tissues and cell types exhibit regulated release of ATP, which, in many cases, is accompanied by the release of uridine nucleotides. Given the relevance of extracellular nucleotide/nucleoside-evoked responses, understanding how ATP and other nucleotides are released from cells is an important physiological question. By facilitating the entry of cytosolic nucleotides into the secretory pathway, recently identified vesicular nucleotide and nucleotide–sugar transporters contribute to the exocytotic release of ATP and UDP-sugars not only from endocrine/exocrine tissues, but also from cell types in which secretory granules have not been biochemically characterized. In addition, plasma membrane connexin hemichannels, pannexin channels, and less-well molecularly defined ATP conducting anion channels have been shown to contribute to the release of ATP (and UTP) under a variety of conditions.




Similar content being viewed by others
Abbreviations
- SLC:
-
Solute carrier
- VNUT:
-
Vesicular nucleotide transporter
- Panx:
-
Pannexin
- Cx:
-
Connexin
- siRNA:
-
Small interfering RNA
- shRNA:
-
Short hairpin RNA
- BAPTA:
-
1,2-Bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DIDS:
-
4,4′-Diisothiocyanostilbene-2,2′-disulfonate
- NBPP:
-
5-Nitro-2-(3-phenylpropylamino)benzoic acid
- fMLP:
-
Formyl-Met-Leu-Phe
References
Burnstock G (2006) Purinergic signalling. Br J Pharmacol 147(Suppl 1):S172–S181
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–532
von Kügelgen I, Harden TK (2011) Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol 61:373–415
Gessi S, Merighi S, Varani K, Borea PA (2011) Adenosine receptors in health and disease. Adv Pharmacol 61:41–75
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694
Corriden R, Insel PA (2010) Basal release of ATP: an autocrine–paracrine mechanism for cell regulation. Sci Signal 3:re1–re25
Praetorius HA, Leipziger J (2009) ATP release from non-excitable cells. Purinergic Signal 5:433–446
Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64:785–795
Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM (2003) Is ATP a signaling agent in plants? Plant Physiol 133:456–461
Chara O, Espelt MV, Krumschnabel G, Schwarzbaum PJ (2011) Regulatory volume decrease and P receptor signaling in fish cells: mechanisms, physiology, and modeling approaches. J Exp Zool A Ecol Genet Physiol 315:175–202
Kukulski F, Levesque SA, Sevigny J (2011) Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol 61:263–299
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430
Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM (2011) Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 61:221–261
Zimmermann H (2008) ATP and acetylcholine, equal brethren. Neurochem Int 52:634–648
Evans RJ, Derkach V, Surprenant A (1992) ATP mediates fast synaptic transmission in mammalian neurons. Nature 357:503–505
Evans RJ, Surprenant A (1992) Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol 106:242–249
Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139
Dean GE, Fishkes H, Nelson PJ, Rudnick G (1984) The hydrogen ion-pumping adenosine triphosphatase of platelet dense granule membrane. Differences from F1F0- and phosphoenzyme-type ATPases. J Biol Chem 259:9569–9574
Gualix J, Abal M, Pintor J, Garcia-Carmona F, Miras-Portugal MT (1996) Nucleotide vesicular transporter of bovine chromaffin granules. Evidence for a mnemonic regulation. J Biol Chem 271:1957–1965
Gualix J, Pintor J, Miras-Portugal MT (1999) Characterization of nucleotide transport into rat brain synaptic vesicles. J Neurochem 73:1098–1104
Kanner BI, Schuldiner S (1987) Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem 22:1–38
Anderson P, Rohlich P, Slorach SA, Uvnas B (1974) Morphology and storage properties of rat mast cell granules isolated by different methods. Acta Physiol Scand 91:145–153
Sorensen CE, Novak I (2001) Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276:32925–32932
Aberer W, Kostron H, Huber E, Winkler H (1978) A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J 172:353–360
Winkler H (1976) The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience 1:65–80
Bankston LA, Guidotti G (1996) Characterization of ATP transport into chromaffin granule ghosts—synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem 271:17132–17138
Hanada H, Moriyama Y, Maeda M, Futai M (1990) Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun 170:873–878
Burgoyne RD, Morgan A (2003) Secretory granule exocytosis. Physiol Rev 83:581–632
Chapman ER, An S, Barton N, Jahn R (1994) SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem 269:27427–27432
Li JY, Jahn R, Dahlstrom A (1996) Axonal transport and targeting of the t-SNAREs SNAP-25 and syntaxin 1 in the peripheral nervous system. Eur J Cell Biol 70:12–22
Rettig J, Neher E (2002) Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science 298:781–785
Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF (2002) Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34:599–611
Chaineau M, Danglot L, Galli T (2009) Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett 583:3817–3826
Reimer RJ, Edwards RH (2004) Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch 447:629–635
Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R (2010) Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics 11:17
Fredriksson R, Nordstrom KJ, Stephansson O, Hagglund MG, Schioth HB (2008) The solute carrier (SLC) complement of the human genome: phylogenetic classification reveals four major families. FEBS Lett 582:3811–3816
Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y (2008) Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A 105:5683–5686
Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S (2010) Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem 285:17406–17416
Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H (2009) Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun 388:1–5
Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M (2011) Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol 589:3471–3482
Novak I (2008) Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal 4:237–253
Chander A, Johnson RG, Reicherter J, Fisher AB (1986) Lung lamellar bodies maintain an acidic internal pH. J Biol Chem 261:6126–6131
Costa JL, Fay DD, Kirk KL (1984) Quinacrine and basic amines in human platelets: subcellular compartmentation and effects on serotonin. Res Commun Chem Pathol Pharmacol 43:25–42
Di A, Krupa B, Bindokas VP, Chen Y, Brown ME, Palfrey HC, Naren AP, Kirk KL, Nelson DJ (2002) Quantal release of free radicals during exocytosis of phagosomes. Nat Cell Biol 4:279–285
Kolber MA, Henkart PA (1988) Quantitation of secretion by rat basophilic leukemia cells by measurements of quinacrine uptake. Biochim Biophys Acta 939:459–466
Goren MB, Swendsen CL, Fiscus J, Miranti C (1984) Fluorescent markers for studying phagosome-lysosome fusion. J Leukoc Biol 36:273–292
Haanes KA, Novak I (2010) ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J 429:303–311
Lazarowski ER, Boucher RC (2009) Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9:262–267
Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER (2007) Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584:245–259
Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC (2011) Coupled nucleotide and mucin hypersecretion from goblet cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45:253–260
Kreda SM, Seminario-Vidal L, van Heusden CA, O'Neal W, Jones L, Boucher RC, Lazarowski ER (2010) Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 588:2255–2267
Leitner JW, Sussman KE, Vatter AE, Schneider FH (1975) Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology 96:662–677
Hutton JC, Penn EJ, Peshavaria M (1983) Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J 210:297–305
Detimary P, Jonas JC, Henquin JC (1996) Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology 137:4671–4676
Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S (2005) Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci 118:4271–4282
Karanauskaite J, Hoppa MB, Braun M, Galvanovskis J, Rorsman P (2009) Quantal ATP release in rat beta-cells by exocytosis of insulin-containing LDCVs. Pflugers Arch 458:389–401
Bulanova E, Bulfone-Paus S (2010) P2 receptor-mediated signaling in mast cell biology. Purinergic Signal 6:3–17
Osipchuk Y, Cahalan M (1992) Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature 359:241–244
Uvnas B (1974) The molecular basis for the storage and release of histamine in rat mast cell granules. Life Sci 14:2355–2366
Smith-Garvin JE, Koretzky GA, Jordan MS (2009) T cell activation. Annu Rev Immunol 27:591–619
Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1:ra6
Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG (2009) Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 23:1685–1693
Tsukimoto M, Tokunaga A, Harada H, Kojima S (2009) Blockade of murine T cell activation by antagonists of P2Y6 and P2X7 receptors. Biochem Biophys Res Commun 384:512–518
Gatof D, Kilic G, Fitz JG (2004) Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol 286:G538–G546
Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP (2011) Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem 286:25363–25376
Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354–1362
Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R (2007) Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282:28749–28758
Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S (2007) Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol 9:945–953
Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310:113–116
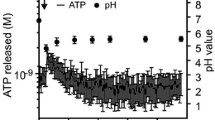
Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575
Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG (2010) Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem 285:8138–8147
Dolovcak S, Waldrop SL, Fitz JG, Kilic G (2009) 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) stimulates cellular ATP release through exocytosis of ATP-enriched vesicles. J Biol Chem 284:33894–33903
Boudreault F, Grygorczyk R (2004) Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561:499–513
Groulx N, Boudreault F, Orlov SN, Grygorczyk R (2006) Membrane reserves and hypotonic cell swelling. J Membr Biol 214:43–56
Tatur S, Groulx N, Orlov SN, Grygorczyk R (2007) Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J Physiol 584:419–435
van der Wijk T, Tomassen SF, Houtsmuller AB, de Jonge HR, Tilly BC (2003) Increased vesicle recycling in response to osmotic cell swelling. Cause and consequence of hypotonicity-provoked ATP release. J Biol Chem 278:40020–40025
Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R (2012) Imaging exocytosis of ATP-containing vesicles with TIRF 1 microscopy in lung epithelial A549 cells. Purinergic Signal 8:59–70
Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol 220:155–162
Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275:10767–10771
Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res 118:10–23
Scrivens M, Dickenson JM (2006) Functional expression of the P2Y(14) receptor in human neutrophils. Eur J Pharmacol 543:166–173
Gao ZG, Ding Y, Jacobson KA (2010) UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol 79:873–879
Sesma JI, Lazarowski ER, Harden TK (2011) UDP-glucose promotes Rho activation in human neutrophils. FASEB J 25:751.16 (Abstr.)
Lazarowski ER, Shea DA, Boucher RC, Harden TK (2003) Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63:1190–1197
Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER (2008) Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol 153:1528–1537
Sesma JI, Esther CR Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER (2009) ER/golgi nucelotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284:12572–12583
Ishida N, Kawakita M (2004) Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch 447:768–775
Guillen E, Hirschberg CB (1995) Transport of adenosine triphosphate into endoplasmic reticulum proteoliposomes. Biochemistry 34:5472–5476
Hirschberg CB, Robbins PW, Abeijon C (1998) Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem 67:49–69
Schwiebert EM (1999) ABC transporter-facilitated ATP conductive transport. Am J Physiol 276:C1–C8
Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG (2001) Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J Membr Biol 183:165–173
Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR (1996) Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science 271:1876–1879
Grygorczyk R, Tabcharani JA, Hanrahan JW (1996) CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol 151:139–148
Watt WC, Lazarowski ER, Boucher RC (1998) Cystic fibrosis transmembrane regulator-independent release of ATP—its implications for the regulation of P2Y(2) receptors in airway epithelia. J Biol Chem 273:14053–14058
Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281:22992–23002
Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC (2004) Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124:513–526
Sabirov R, Okada Y (2005) ATP release via anion channels. Purinergic Signal 1:311–328
Sabirov RZ, Okada Y (2009) The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci 59:3–21
Sabirov RZ, Dutta AK, Okada Y (2001) Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol 118:251–266
Sabirov RZ, Okada Y (2004) Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J 87:1672–1685
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M (2002) Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 119:511–520
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587:2141–2149
Blum AE, Walsh BC, Dubyak GR (2010) Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells. Am J Physiol Cell Physiol 298:C386–C396
Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y (1999) Swelling-induced, CFTR-independent ATP release from a human epithelial cell line. J Gen Physiol 114:525–533
Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y (2009) Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol 587:2197–2209
Suzuki M, Mizuno A (2004) A novel human Cl(−) channel family related to Drosophila flightless locus. J Biol Chem 279:22461–22468
Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal 3:ra45
Scemes E, Spray DC, Meda P (2009) Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch 457:1207–1226
D'hondt C, Ponsaerts R, De SH, Vinken M, De VE, De BM, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G (2011) Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal 23:305–316
Thompson RJ, MacVicar BA (2008) Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin) 2:81–86
D'hondt C, Ponsaerts R, De SH, Bultynck G, Himpens B (2009) Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays 31:953–974
Nakagawa S, Maeda S, Tsukihara T (2010) Structural and functional studies of gap junction channels. Curr Opin Struct Biol 20:423–430
Wang J, Ma M, Locovei S, Keane RW, Dahl G (2007) Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293:C1112–C1119
Muller DJ, Hand GM, Engel A, Sosinsky GE (2002) Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J 21:3598–3607
Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M (1998) Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A 95:15735–15740
Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M (2002) Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A 99:9840–9845
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M (2008) Connexin 43 hemichannels are permeable to ATP. J Neurosci 28:4702–4711
Romanello M, Pani B, Bicego M, D'Andrea P (2001) Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289:1275–1281
De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L (2005) Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25:34–44
De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L (2009) Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46:176–187
Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J (2008) Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294:R1769–R1776
Schock SC, Leblanc D, Hakim AM, Thompson CS (2008) ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun 368:138–144
Huckstepp RT, Id BR, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N (2010) Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588:3901–3920
Funk GD (2010) The ‘connexin’ between astrocytes, ATP and central respiratory chemoreception. J Physiol 588:4335–4337
Dale N, Frenguelli BG (2009) Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol 7:160–179
Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A 105:18770–18775
Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J (2009) Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20:1724–1732
Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD (2011) Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286:1054–1060
Eltzschig HK, Macmanus CF, Colgan SP (2008) Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med 18:103–107
Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP (2006) ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99:1100–1108
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731
Singh D, Lampe PD (2003) Identification of connexin-43 interacting proteins. Cell Commun Adhes 10:215–220
Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, Pointis G (2008) Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci 121:4069–4078
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2010) The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res 70:4222–4232
Scemes E (2008) Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia 56:145–153
Spray DC, Iacobas DA (2007) Organizational principles of the connexin-related brain transcriptome. J Membr Biol 218:39–47
Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC (2008) Similar transcriptomic alterations in Cx43 knockdown and knockout astrocytes. Cell Commun Adhes 15:195–206
Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE (2010) Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem 285:24420–24431
Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120:3772–3783
Boassa D, Qiu F, Dahl G, Sosinsky G (2008) Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes 15:119–132
Bruzzone R, Barbe MT, Jakob NJ, Monyer H (2005) Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92:1033–1043
Dahl G, Locovei S (2006) Pannexin: to gap or not to gap, is that a question? IUBMB Life 58:409–419
Ma W, Hui H, Pelegrin P, Surprenant A (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328:409–418
Silverman W, Locovei S, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–C767
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082
Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68
Locovei S, Bao L, Dahl G (2006) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A 103:7655–7659
Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M (2009) Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41:525–534
Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER (2011) Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286:26277–26286
Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER (2009) Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J Biol Chem 284:20638–20648
Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS (2010) Pannexin 1 is the conduit for low oxygen tension-induced atp release from human erythrocytes. Am J Physiol Heart Circ Physiol 299:H1146–H1152
Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ (2011) Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med 39:2478–2486
Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA (2011) Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci U S A 108:10986–10991
Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM (2010) Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol 299:C1308–C1317
Reigada D, Lu W, Zhang M, Mitchell CH (2008) Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience 157:396–404
Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM (2011) Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol (in press)
Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E (2009) ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem 284:34490–34505
Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007) The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104:6436–6441
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H (2008) P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J 27:3104–3115
Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Metivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, Kroemer G, Perfettini JL (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med 208:1823–1834
Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097
Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE (2011) Pannexin1 regulates {alpha}1-adrenergic receptor-mediated vasoconstriction. Circ Res 109:80–85
Qiu F, Wang J, Spray DC, Scemes E, Dahl G (2011) Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585:3430–3435
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286
Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM (2011) Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186:6553–6561
Locovei S, Wang J, Dahl G (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580:239–244
Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G (2011) Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem 286:290–298
Qiu F, Dahl G (2009) A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol 296:C250–C255
Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R (2009) The potassium channel subunit Kvbeta3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. FEBS J 276:6258–6270
Bunse S, Schmidt M, Prochnow N, Zoidl G, Dermietzel R (2010) Intracellular cysteine 346 is essentially involved in regulating Panx1 channel activity. J Biol Chem 285:38444–38452
Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW (2010) Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem 285:9147–9160
Thompson RJ, Zhou N, MacVicar BA (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927
Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA (2008) Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–1559
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581:483–488
Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW (2008) Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes 15:133–142
Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y (2010) Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem 285:18948–18958
Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y (2011) Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol 193:1257–1274
Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–321
Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 519(Pt 2):335–346
Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F (2005) A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell 16:3659–3665
Suadicani SO, Brosnan CF, Scemes E (2006) P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26:1378–1385
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
O'Neil RG, Heller S (2005) The mechanosensitive nature of TRPV channels. Pflugers Arch 451:193–203
Wu L, Gao X, Brown RC, Heller S, O'Neil RG (2007) Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293:F1699–F1713
Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284:21257–21264
Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453–3462
Silva GB, Garvin JL (2008) TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295:F1090–F1095
Acknowledgments
We thank Lisa Brown for editorial assistance of the manuscript. Supported by National Institute of Health grant P01-HL034322.
Conflict of interest statement
The author has no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signalling 8, 359–373 (2012). https://doi.org/10.1007/s11302-012-9304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-012-9304-9