Abstract
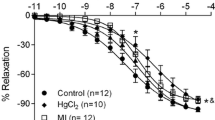
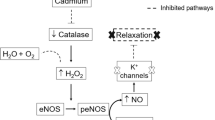
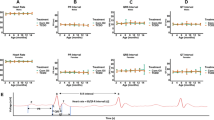
The mechanisms by which 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) increases the incidence of human cardiovascular disease are not known. We investigated the degree to which cardiovascular disease develops in mice following subchronic TCDD exposure. Adult male C57BL/6 mice were dosed with vehicle or 300 ng TCDD/kg by oral gavage three times per week for 60 days. Blood pressure was recorded by radiotelemetry and aortic endothelial function was assessed by acetylcholine-induced vasorelaxation. Mean arterial pressure of TCDD-exposed mice was increased significantly by day 4 and between days 7–10, 25–35, and 45–60 with two periods of normalization on days 11–24 and days 36–39. Consistent with a prolonged period of systemic hypertension, heart weight was increased and was associated with concentric left ventricular hypertrophy. Significant increases in superoxide production also were observed in the kidney, heart, and aorta of TCDD-exposed mice. Furthermore, increased aortic superoxide resulted in endothelial dysfunction as demonstrated by significant impairment of acetylcholine-induced vasorelaxation in TCDD-exposed mice, which was restored by tempol, a superoxide dismutase (SOD) mimetic. Our model is the first to definitely demonstrate that sustained AhR activation by TCDD increases blood pressure and induces cardiac hypertrophy, which may be mediated, in part, by increased superoxide.






Similar content being viewed by others
References
ATSDR. (1998). Toxicological profile for chlorinated dibenzo-p-dioxins. Atlanta: Agency for Toxic Substance and Disease Registry.
Swanson, H. I., & Bradfield, C. A. (1993). The AH-receptor: Genetics, structure and function. Pharmacogenetics, 3, 213–230. doi:10.1097/00008571-199310000-00001.
Fernandez-Salguero, P. M., Hilbert, D. M., Rudikoff, S., Ward, J. M., & Gonzalez, F. J. (1996). Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicology and Applied Pharmacology, 140, 173–179. doi:10.1006/taap.1996.0210.
Mimura, J., Yamashita, K., Nakamura, K., Morita, M., Takagi, T. N., Nakao, K., et al. (1997). Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes to Cells, 2, 645–654. doi:10.1046/j.1365-2443.1997.1490345.x.
Peters, J. M., Narotsky, M. G., Elizondo, G., Fernandez-Salguero, P. M., Gonzalez, F. J., & Abbott, B. D. (1999). Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicological Sciences, 47, 86–92. doi:10.1093/toxsci/47.1.86.
Kim, J. S., Lim, H. S., Cho, S. I., Cheong, H. K., & Lim, M. K. (2003). Impact of Agent Orange exposure among Korean Vietnam veterans. Industrial Health, 41, 149–157. doi:10.2486/indhealth.41.149.
Kang, H. K., Dalager, N. A., Needham, L. L., Patterson, D. G., Jr., Lees, P. S., Yates, K., et al. (2006). Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. American Journal of Industrial Medicine, 49, 875–884. doi:10.1002/ajim.20385.
Dalton, T. P., Kerzee, J. K., Wang, B., Miller, M., Dieter, M. Z., Lorenz, J. N., et al. (2001). Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovascular Toxicology, 1, 285–298. doi:10.1385/CT:1:4:285.
Jokinen, M. P., Walker, N. J., Brix, A. E., Sells, D. M., Haseman, J. K., & Nyska, A. (2003). Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Cardiovascular Toxicology, 3, 299–310. doi:10.1385/CT:3:4:299.
Brunnberg, S., Andersson, P., Lindstam, M., Paulson, I., Poellinger, L., & Hanberg, A. (2006). The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure-effects in vital organs. Toxicology, 224, 191–201. doi:10.1016/j.tox.2006.04.045.
Touyz, R. M. (2004). Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension, 44, 248–252. doi:10.1161/01.HYP.0000138070.47616.9d.
Slezak, B. P., Hatch, G. E., DeVito, M. J., Diliberto, J. J., Slade, R., Crissman, K., et al. (2000). Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicological Sciences, 54, 390–398. doi:10.1093/toxsci/54.2.390.
Huwe, J. K., & Smith, D. J. (2005). Laboratory and on-farm studies on the bioaccumulation and elimination of dioxins from a contaminated mineral supplement fed to dairy cows. Journal of Agricultural and Food Chemistry, 53, 2362–2370. doi:10.1021/jf0480997.
Simon, P. (2003). Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics (Oxford, England), 19, 1439–1440. doi:10.1093/bioinformatics/btg157.
Lund, A. K., Peterson, S. L., Timmins, G. S., & Walker, M. K. (2005). Endothelin-1-mediated increase in reactive oxygen species and NADPH Oxidase activity in hearts of aryl hydrocarbon receptor (AhR) null mice. Toxicological Sciences, 88, 265–273. doi:10.1093/toxsci/kfi284.
Dikalov, S., Griendling, K. K., & Harrison, D. G. (2007). Measurement of reactive oxygen species in cardiovascular studies. Hypertension, 49, 717–727. doi:10.1161/01.HYP.0000258594.87211.6b.
Diliberto, J. J., DeVito, M. J., Ross, D. G., & Birnbaum, L. S. (2001). Subchronic exposure of [3H]-2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female B6C3F1 mice: Relationship of steady-state levels to disposition and metabolism. Toxicological Sciences, 61, 241–255. doi:10.1093/toxsci/61.2.241.
Levick, J. R. (2003). Introduction to cardiovascular physiology. London: Hodder Arnold.
Itoh, K., Chiba, T., Takahashi, S., Ishii, T., Igarashi, K., Katoh, Y., et al. (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications, 236, 313–322. doi:10.1006/bbrc.1997.6943.
Venugopal, R., & Jaiswal, A. K. (1996). Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proceedings of the National Academy of Sciences of the United States of America, 93, 14960–14965. doi:10.1073/pnas.93.25.14960.
Muscoli, C., Cuzzocrea, S., Riley, D. P., Zweier, J. L., Thiemermann, C., Wang, Z. Q., et al. (2003). On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. British Journal of Pharmacology, 140, 445–460. doi:10.1038/sj.bjp.0705430.
Tsukahara, C., Sugiyama, F., Paigen, B., Kunita, S., & Yagami, K. (2004). Blood pressure in 15 inbred mouse strains and its lack of relation with obesity and insulin resistance in the progeny of an NZO/HILtJ x C3H/HeJ intercross. Mammalian Genome, 15, 943–950. doi:10.1007/s00335-004-2411-3.
Gross, V., & Luft, F. C. (2003). Exercising restraint in measuring blood pressure in conscious mice. Hypertension, 41, 879–881. doi:10.1161/01.HYP.0000060866.69947.D1.
Kario, K., Matsuo, T., Kobayashi, H., Imiya, M., Matsuo, M., & Shimada, K. (1996). Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension, 27, 130–135.
Shimada, K., Kawamoto, A., Matsubayashi, K., Nishinaga, M., Kimura, S., & Ozawa, T. (1992). Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. Journal of Hypertension, 10, 875–878.
Huang, P. L., Huang, Z., Mashimo, H., Bloch, K. D., Moskowitz, M. A., Bevan, J. A., et al. (1995). Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature, 377, 239–242. doi:10.1038/377239a0.
Pollock, D. M. (2005). Endothelin, angiotensin, and oxidative stress in hypertension. Hypertension, 45, 477–480. doi:10.1161/01.HYP.0000158262.11935.d0.
Bagchi, D., Balmoori, J., Bagchi, M., Ye, X., Williams, C. B., & Stohs, S. J. (2002). Comparative effects of TCDD, endrin, naphthalene and chromium (VI) on oxidative stress and tissue damage in the liver and brain tissues of mice. Toxicology, 175, 73–82. doi:10.1016/S0300-483X(02)00062-8.
Hassoun, E. A., Wilt, S. C., DeVito, M. J., Van, B. A., Alsharif, N. Z., Birnbaum, L. S., et al. (1998). Induction of oxidative stress in brain tissues of mice after subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicological Sciences, 42, 23–27.
Hassoun, E. A., Li, F., Abushaban, A., & Stohs, S. J. (2000). The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology, 145, 103–113. doi:10.1016/S0300-483X(99)00221-8.
Chen, H. L., Hsu, C. Y., Hung, D. Z., & Hu, M. L. (2006). Lipid peroxidation and antioxidant status in workers exposed to PCDD/Fs of metal recovery plants. The Science of the Total Environment, 372, 12–19. doi:10.1016/j.scitotenv.2006.06.008.
Alsharif, N. Z., & Hassoun, E. A. (2004). Protective effects of vitamin A and vitamin E succinate against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced body wasting, hepatomegaly, thymic atrophy, production of reactive oxygen species and DNA damage in C57BL/6J mice. Basic & Clinical Pharmacology & Toxicology, 95, 131–138.
Slim, R., Toborek, M., Robertson, L. W., Lehmler, H. J., & Hennig, B. (2000). Cellular glutathione status modulates polychlorinated biphenyl-induced stress response and apoptosis in vascular endothelial cells. Toxicology and Applied Pharmacology, 166, 36–42. doi:10.1006/taap.2000.8944.
Dong, W., Teraoka, H., Yamazaki, K., Tsukiyama, S., Imani, S., Imagawa, T., et al. (2002). 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicological Sciences, 69, 191–201. doi:10.1093/toxsci/69.1.191.
Pelclova, D., Prazny, M., Skrha, J., Fenclova, Z., Kalousova, M., Urban, P., et al. (2007). 2,3,7,8-TCDD exposure, endothelial dysfunction and impaired microvascular reactivity. Human and Experimental Toxicology, 26, 705–713. doi:10.1177/0960327107083971.
Hassoun, E. A., Al-Ghafri, M., & Abushaban, A. (2003). The role of antioxidant enzymes in TCDD-induced oxidative stress in various brain regions of rats after subchronic exposure. Free Radical Biology and Medicine, 35, 1028–1036. doi:10.1016/S0891-5849(03)00458-1.
Lim, J., DeWitt, J. C., Sanders, R. A., Watkins, J. B., III, & Henshel, D. S. (2007). Suppression of endogenous antioxidant enzymes by 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in chicken liver during development. Archives of Environmental Contamination and Toxicology, 52, 590–595. doi:10.1007/s00244-006-0168-2.
Andreyev, A. Y., Kushnareva, Y. E., & Starkov, A. A. (2005). Mitochondrial metabolism of reactive oxygen species. Biochemistry. Biokhimiia, 70, 200–214. doi:10.1007/s10541-005-0102-7.
Puddu, P., Puddu, G. M., Cravero, E., De, P. S., & Muscari, A. (2007). The putative role of mitochondrial dysfunction in hypertension. Clinical and Experimental Hypertension, 29, 427–434. doi:10.1080/10641960701613852.
Senft, A. P., Dalton, T. P., Nebert, D. W., Genter, M. B., Hutchinson, R. J., & Shertzer, H. G. (2002). Dioxin increases reactive oxygen production in mouse liver mitochondria. Toxicology and Applied Pharmacology, 178, 15–21. doi:10.1006/taap.2001.9314.
Genter, M. B., Clay, C. D., Dalton, T. P., Dong, H., Nebert, D. W., & Shertzer, H. G. (2006). Comparison of mouse hepatic mitochondrial versus microsomal cytochromes P450 following TCDD treatment. Biochemical and Biophysical Research Communications, 342, 1375–1381. doi:10.1016/j.bbrc.2006.02.121.
Verhaar, M. C., Westerweel, P. E., van Zonneveld, A. J., & Rabelink, T. J. (2004). Free radical production by dysfunctional eNOS. Heart (British Cardiac Society), 90, 494–495. doi:10.1136/hrt.2003.029405.
Moens, A. L., & Kass, D. A. (2006). Tetrahydrobiopterin and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 2439–2444. doi:10.1161/01.ATV.0000243924.00970.cb.
Mitchell, B. M., Dorrance, A. M., & Webb, R. C. (2003). GTP cyclohydrolase 1 inhibition attenuates vasodilation and increases blood pressure in rats. American Journal of Physiology. Heart and Circulatory Physiology, 285, H2165–H2170.
Mitchell, B. M., Dorrance, A. M., & Webb, R. C. (2003). GTP cyclohydrolase 1 downregulation contributes to glucocorticoid hypertension in rats. Hypertension, 41, 669–674. doi:10.1161/01.HYP.0000051889.62249.5D.
Landmesser, U., Dikalov, S., Price, S. R., McCann, L., Fukai, T., Holland, S. M., et al. (2003). Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. The Journal of Clinical Investigation, 111, 1201–1209.
Andreasen, E. A., Mathew, L. K., & Tanguay, R. L. (2006). Regenerative growth is impacted by TCDD: Gene expression analysis reveals extracellular matrix modulation. Toxicological Sciences, 92, 254–269. doi:10.1093/toxsci/kfj118.
Carney, S. A., Chen, J., Burns, C. G., Xiong, K. M., Peterson, R. E., & Heideman, W. (2006). Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Molecular Pharmacology, 70, 549–561. doi:10.1124/mol.106.025304.
Puntarulo, S., & Cederbaum, A. I. (1998). Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radical Biology and Medicine, 24, 1324–1330. doi:10.1016/S0891-5849(97)00463-2.
Shertzer, H. G., Clay, C. D., Genter, M. B., Chames, M. C., Schneider, S. N., Oakley, G. G., et al. (2004). Uncoupling-mediated generation of reactive oxygen by halogenated aromatic hydrocarbons in mouse liver microsomes. Free Radical Biology and Medicine, 36, 618–631. doi:10.1016/j.freeradbiomed.2003.11.014.
Annas, A., & Brittebo, E. B. (1998). Localization of cytochrome P4501A1 and covalent binding of a mutagenic heterocyclic amine in blood vessel endothelia of rodents. Toxicology, 129, 145–156. doi:10.1016/S0300-483X(98)00087-0.
Teraoka, H., Dong, W., Tsujimoto, Y., Iwasa, H., Endoh, D., Ueno, N., et al. (2003). Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochemical and Biophysical Research Communications, 304, 223–228. doi:10.1016/S0006-291X(03)00576-X.
Kaplan, N. M. (2002). In N. M. Kaplan (Ed.), Kaplan’s clinical hypertension (pp. 56–135). Philadelphia: Lippincott Williams & Wilkins.
Schiffrin, E. L. (2005). Vascular endothelin in hypertension. Vascular Pharmacology, 43, 19–29. doi:10.1016/j.vph.2005.03.004.
Diliberto, J. J., Burgin, D., & Birnbaum, L. S. (1999). Effects of CYP1A2 on disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,4,7,8-pentachlorodibenzofuran, and 2,2′,4,4′,5,5′-hexachlorobiphenyl in CYP1A2 knockout and parental (C57BL6N and 129/Sv) strains of mice. Toxicology and Applied Pharmacology, 159, 52–64. doi:10.1006/taap.1999.8720.
Acknowledgments
Funding National Institute of Health (ES12335 and HL078914 to MKW, ES12072 to UNM, 5T32HL007736 to UNM). Phillip Kopf is recognized as an American Foundation for Pharmaceutical Education Pre-Doctoral Fellow. The authors thank Drs. Nancy L. Kanagy and Matthew J. Campen for their valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Rights and permissions
About this article
Cite this article
Kopf, P.G., Huwe, J.K. & Walker, M.K. Hypertension, Cardiac Hypertrophy, and Impaired Vascular Relaxation Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin are Associated with Increased Superoxide. Cardiovasc Toxicol 8, 181–193 (2008). https://doi.org/10.1007/s12012-008-9027-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-008-9027-x