Abstract
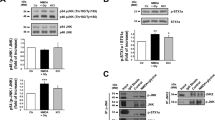
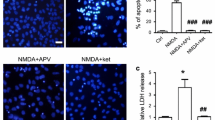
N-methyl-d-aspartate (NMDA) receptors play a key role in excitatory synaptic transmission, plasticity and neural development, and they also mediate excitotoxicity that is involved in both acute neuronal damage and chronic neurodegenerative diseases. Regulation of the NMDA channel activity is critical for the pathological processes of these diseases. The canonical transient receptor potential channels (TRPCs) are Ca2+-permeable nonselective cation channels with various physiological functions, including promoting neuronal survival. Here, we reported that TRPC6, a member of the TRPC family, inhibited the NMDA-induced current in primary cultured hippocampal neurons. Overexpression of TRPC6 or application of 1-oleoyl-2-acetyl-sn-glycerol, a compound known to activate TRPCs, inhibited the NMDA-induced current in these neurons assayed by the whole-cell patch-clamp recording. Consistently, downregulation of TRPC6 or application of SKF96365, a compound known to inhibit TRPCs, enhanced this current. The peak amplitude of the NMDA current in the neurons isolated from TRPC6 transgenic mice was greatly suppressed than that in the neurons isolated from the wild-type littermates. Furthermore, TRPC6 might activate calcineurin to inhibit the activity of NMDA receptors in cultured hippocampal neurons. Together, these results suggested that TRPC6 might be a novel negative modulator of NMDA receptors.




Similar content being viewed by others
References
Albert, A. P., & Large, W. A. (2003). Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. Journal of Physiology, 552(Pt 3), 789–795.
Blandini, F., Greenamyre, J. T., & Nappi, G. (1996). The role of glutamate in the pathophysiology of Parkinson’s disease. Functional Neurology, 11, 3–15.
Burnashev, N., Zhou, Z., Neher, E., & Sakmann, B. (1995). Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. Journal of Physiology, 485(Pt 2), 403–418.
Clapham, D. E. (2003). TRP channels as cellular sensors. Nature, 426, 517–524.
Du, W., Huang, J., Yao, H., Zhou, K., Duan, B., & Wang, Y. (2010). Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. The Journal of clinical investigation, 120, 3480–3492.
Dumont, F. J. (2000). FK506, an immunosuppressant targeting calcineurin function. Current Medicinal Chemistry, 7(7), 731–748.
Garaschuk, O., Schneggenburger, R., Schirra, C., Tempia, F., & Konnerth, A. (1996). Fractional Ca2+ currents through somatic and dendritic glutamate receptor channels of rat hippocampal CA1 pyramidal neurones. Journal of Physiology, 491(Pt 3), 757–772.
Greengard, P. (2001). The neurobiology of slow synaptic transmission. Science, 294, 1024–1030.
Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T., & Schultz, G. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature, 397, 259–263.
Inoue, R., Okada, T., Onoue, H., Hara, Y., Shimizu, S., Naitoh, S., et al. (2001). The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2 +)-permeable cation channel. Circulation Research, 88, 325–332.
Jia, Y., Zhou, J., Tai, Y., & Wang, Y. (2007). TRPC channels promote cerebellar granule neuron survival. Nature Neuroscience, 10, 559–567.
Kiyonaka, S., Kato, K., Nishida, M., Mio, K., Numaga, T., Sawaguchi, Y., et al. (2009). Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proceedings of the National Academy of Sciences of the United States of America, 106, 5400–5405.
Kutsuwada, T., Kashiwabuchi, N., Mori, H., Sakimura, K., Kushiya, E., Araki, K., et al. (1992). Molecular diversity of the NMDA receptor channel. Nature, 358, 36–41.
Kuwahara, K., Wang, Y., McAnally, J., Richardson, J. A., Bassel-Duby, R., Hill, J. A., et al. (2006). TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. The Journal of clinical investigation, 116, 3114–3126.
Lee, F. J., Xue, S., Pei, L., Vukusic, B., Chery, N., Wang, Y., et al. (2002). Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell, 111, 219–230.
Lee, J. M., Zipfel, G. J., & Choi, D. W. (1999). The changing landscape of ischaemic brain injury mechanisms. Nature, 399, A7–A14.
Mayer, M. L., & Westbrook, G. L. (1987). Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology, 394, 501–527.
Meguro, H., Mori, H., Araki, K., Kushiya, E., Kutsuwada, T., Yamazaki, M., et al. (1992). Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature, 357, 70–74.
Meldrum, B., & Garthwaite, J. (1990). Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends in Pharmacological Sciences, 11, 379–387.
Merritt, J. E., Armstrong, W. P., Benham, C. D., Hallam, T. J., Jacob, R., Jaxa-Chamiec, A., et al. (1990). SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Journal of Biochemistry, 271, 515–522.
Monyer, H., Sprengel, R., Schoepfer, R., Herb, A., Higuchi, M., Lomeli, H., et al. (1992). Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science, 256, 1217–1221.
Rogers, M., & Dani, J. A. (1995). Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophysical Journal, 68, 501–506.
Saleh, S. N., Albert, A. P., & Large, W. A. (2009). Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. Journal of Physiology, 587, 5361–5375.
Schneggenburger, R., Zhou, Z., Konnerth, A., & Neher, E. (1993). Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron, 11, 133–143.
Skeberdis, V. A., Chevaleyre, V., Lau, C. G., Goldberg, J. H., Pettit, D. L., Suadicani, S. O., et al. (2006). Protein kinase A regulates calcium permeability of NMDA receptors. Nature Neuroscience, 9, 501–510.
Tong, G., Shepherd, D., & Jahr, C. E. (1995). Synaptic desensitization of NMDA receptors by calcineurin. Science, 267, 1510–1512.
Tu, W., Xu, X., Peng, L., Zhong, X., Zhang, W., Soundarapandian, M. M., et al. (2010). DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell, 140, 222–234.
Wang, J., Liu, S., Fu, Y., Wang, J. H., & Lu, Y. (2003). Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nature Neuroscience, 6, 1039–1047.
Zhang, J. M., Wang, H. K., Ye, C. Q., Ge, W., Chen, Y., Jiang, Z. L., et al. (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron, 40, 971–982.
Zhou, J., Du, W., Zhou, K., Tai, Y., Yao, H., Jia, Y., et al. (2008). Critical role of TRPC6 channels in the formation of excitatory synapses. Nature Neuroscience, 11, 741–743.
Acknowledgments
We thank Professor MX. Zhu for constructs, and we thank Professor Yizheng Wang for TRPC6 transgenic mice. This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and National Natural Science Foundation of China (NSFC 81073079).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, H., Pan, J., Pan, L. et al. TRPC6 Inhibited NMDA Current in Cultured Hippocampal Neurons. Neuromol Med 15, 389–395 (2013). https://doi.org/10.1007/s12017-013-8226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8226-1