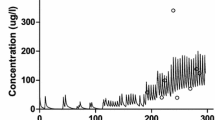
Abstract
Purpose. The objective of this study was to evaluate the effect of a potent P-gp inhibitor, GF120918, on the systemic pharmacokinetics and antinociceptive pharmacodynamics of a single intravenous dose of morphine in rats.
Methods. Male Sprague-Dawley rats received either 500 mg base/kg/d GF120918 or vehicle for 4 days by gavage, or no pretreatment. On day 4, morphine was administered as a 1- or 2-mg/kg i.v. bolus. Antinociception, expressed as percent of maximum possible response (%MPR), was evaluated over 300 min after morphine administration. Serial blood samples were collected and analyzed for morphine and morphine-3-glucuronide (M3G) by HPLC.
Results. Morphine clearance and distribution volume were not altered significantly by GF120918. M3G AUC in the GF120918-treated rats was approximately 2-fold higher than in vehicle-treated rats. For both morphine doses, %MPR and the area under the effect-time curve at 300 min were significantly higher in the GF120918-treated rats. A pharmacokinetic/pharmacodynamic effect model accurately described the effect-concentration data for the rats that received 1-mg/kg morphine; ke0 was significantly smaller for GF 120918- vs. vehicle-treated and control rats (0.060 ± 0.028 vs. 0.228 ± 0.101 vs. 0.274 ± 0.026 min−1, p=0.0023). EC50 and γ were similar between treatment groups.
Conclusions. Pretreatment with GF 120918 enhanced morphine antinociception, as assessed by the hot-lamp tail-flick assay, and elevated systemic M3G concentrations in rats. The differential pharmacologic response to morphine in the GF120918-treated animals could not be attributed to alterations in systemic morphine pharmacokinetics.
Similar content being viewed by others
REFERENCES
C. Cordon-Cardo, J. P. O'Brien, J. Boccia, D. Casals, J. R. Bertino, and M. R. Melamed. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 38:1277–1287 (1990).
F. Thiebaut, T. Tsuruo, H. Hamada, M. M. Gottesman, I. Pastan, and M. C. Willingham. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 84:7735–7738 (1987).
I. Pastan and M. M. Gottesman. Multidrug resistance. Annu Rev. Med. 42:277–286 (1991).
H. L. Pearce, A. R. Safa, N. J. Bach, M. A. Winter, M. C. Cirtain, and W. T. Beck. Essential features of the P-glycoprotein pharmacophore as defined by a series of reserpine analogs that modulate multidrug resistance. Proc. Natl. Acad. Sci. USA 86:5128–5132 (1989).
J. M. Zamora, H. L. Pearce, and W. T. Beck. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells. Mol. Pharmacol. 33:454–462 (1988).
J. J. Kaufman, N. M. Semo, and W. S. Koski. Microelectrometric titration measurement of the pK's and partition and drug distribution coefficients of narcotics and narcotic antagonists and their pH and temperature dependence. J. Med. Chem. 18:647–655 (1975).
R. Callaghan and J. R. Riordan. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J. Biol. Chem. 268:16059–16064 (1993).
A. H. Schinkel, E. Wagenaar, L. van Deemter, C. A. A. M. Mol, and P. Borst. Absence of the mdr la P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 96:1698–1705 (1995).
L. J. Goldstein, I. Pastan, and M. M. Gottesman. Multidrug resistance in human cancer. Crit. Rev. Oncol. Hematol. 12:243–253 (1992).
F. Hyafil, C. Vergely, P. Du Vignaud, and T. Grand-Perret. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 53:4595–4602 (1993).
S. M. Witherspoon, D. L. Emerson, B. M. Kerr, T. L. Lloyd, W. S. Dalton, and P. S. Wissel. Flow cytometric assay of modulation of P-glycoprotein function in whole blood by the multidrug resistance inhibitor GG918. Clin. Cancer Res. 2:7–12 (1996).
P. A. Glare, T. D. Walsh, and C. E. Pippenger. A simple method for the simultaneous determination of morphine and its principal metabolites in plasma using high-performance liquid chromatography and fluorometric detection. Ther. Drug Monit. 13:226–232 (1991).
R. F. Venn and A. Michalkiewcz. Fast reliable assay for morphine and its metabolites using high-performance liquid chromatography and native fluoresence detection. J. Chromatogr. 525:379–388 (1990).
D. M. Ouellet and G. M. Pollack. Biliary excretion and enterohepatic recirculation of morphine-3-glucuronide in rats. Drug Metab. Dispos. 23:478–484 (1995).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd edition, Marcel Dekker, New York, 1982.
L. B. Sheiner, D. R. Stanski, S. Vozeh, R. D. Miller, and J. Ham. Simultaneous modeling of pharmacokinetics and pharmacodynamics: applications to d-tubocuranine. Clin. Pharmacol. Ther. 25:358–371 (1979).
J. H. Jaffe and W. R. Martin. Opioid analgesics and antagonists. In A. G. Gilman, T. W. Rall, A. S. Nies, and P. Taylor (eds.), Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th edition, Pergamon Press, New York, 1990, pp. 485–521.
A. Sakata, I. Tamai, K. Kawazu, Y. Deguchi, T. Ohnishi, A Saheki, and A Tsuji. In vivo evidence for ATP-dependent and P-glycoprotein-mediated transport of cyclosporin A at the blood-brain barrier. Biochem. Pharmacol. 48:1989–1992 (1994).
A. Tsuji, T. Tersaki, Y. Takabatake, Y. Tenda, I. Tamai, T. Yamashima, S. Moritani, T. Tsuruo, and J. Yamashita. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 51:1427–1437 (1992).
E. G. Chikhale, P. S. Burton, and R. T. Borchardt. The effect of verapamil on the transport of peptides across the blood-brain barrier in rats: kinetic evidence for an apically polarized efflux mechanism. J. Pharmacol. Exp. Ther. 273:298–303 (1995).
T. Tatsuta, M. Naito, T. Oh-hara, I. Sugawara, and T. Tsuruo. Functional involvement of P-glycoprotein in blood-brain barrier. J. Biol. Chem. 267:20383–20391–223 (1992).
R. W. Milne, R. L. Nation, and A. A. Somogyi. The disposition of morphine and its 3-and 6-glucuronide metabolites in humans and animals, and the importance of the metabolites to the pharmacological effects of morphine. Drug Metab. Rev. 28:345–472 (1996).
T. L. Horton and G. M. Pollack. Enterohepatic recirculation and metabolism of morphine in the rat. J. Pharm. Sci. 80:1147–1152 (1991).
M. Gosland, C. Tsuboi, T. Hoffman, S. Goodin, and M. Vore. 17 β-estradiol glucuronide: an inducer of cholestasis and a physiological substrate for the multidrug resistance transporter. Cancer Res. 53:5382–5385 (1993).
Y. Liu, L. Huang, T. Hoffman, M. Gosland, and M. Vore. MDRI substrates/modulators protect against β-estradiol-17β-D-glucuronide cholestasis in rat liver. Cancer Res. 56:4992–4997 (1996).
J. Huwyler, J. Drewe, C. Klusemann, and G. Fricker. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br. J. Pharmacol. 118:1879–1885 (1996).
K. Hewett, A. H. Dickenson, and H. J. McQuay. Lack of effect of morphine-3-glucuronide on the spinal antinociceptive actions of morphine in the rat: an electrophysiological study. Pain 53:59–63 (1993).
D. M. Ouellet and G. M. Pollack. Effect of prior morphine-3-glucuronide exposure on morphine disposition and antinociception. Biochem. Pharmacol. 53:1451–1457 (1997).
N. Suzuki, E. Kalso E., and P. H. Rosenberg. Intrathecal morphine-3-glucuronide does not antagonize spinal antinociception by morphine or morphine-6-glucuronide in rats. Eur. J. Pharmacol. 249:247–250 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Letrent, S.P., Pollack, G.M., Brouwer, K.R. et al. Effect of GF120918, a Potent P-glycoprotein Inhibitor, on Morphine Pharmacokinetics and Pharmacodynamics in the Rat. Pharm Res 15, 599–605 (1998). https://doi.org/10.1023/A:1011938112599
Issue Date:
DOI: https://doi.org/10.1023/A:1011938112599