Abstract
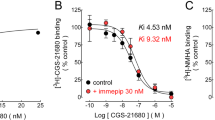
Binding properties of the subtypes of adenosine A2 receptors in membrane preparations and the effects of adenosine receptor ligands on cAMP accumulation in slices from the optic tectum of neonatal chicks have been investigated. [3H]2-[4-(2-p-carboxyethyl)phenylamino]-5'-N-ethylcarboxaminoadenosine (CGS 21680), a selective ligand for adenosine A2a receptors, did not bind to optic tectal membranes, as observed with rat striatal membranes. CGS 21680 also did not induce cyclic AMP accumulation in optic tectum slices. However, 5'-N-ethylcarboxamidoadenosine (NECA), 2-chloro-adenosine or adenosine induced a 2.5- to 3-fold increase on cyclic AMP accumulation in this preparation. [3H]NECA binds to fresh non-washed-membranes obtained from optic tectum of chicks, displaying one population of binding sites, which can be displaced by NECA, 8-phenyltheophylline, 2-chloro-adenosine, but is not affected by CGS 21680. The estimated KD value was 400.90 ± 80.50 nM and the Bmax was estimated to be 2.51 ± 0.54 pmol/mg protein. Guanine nucleotides, which modulate G-proteins activity intracellularly, are also involved in the inhibition of glutamate responses by acting extracellularly. Moreover, we have previously reported that guanine nucleotides potentiate, while glutamate inhibits, adenosine-induced cyclic AMP accumulation in slices from optic tectum of chicks. However, the guanine nucleotides, GMP or GppNHp and the metabotropic glutamate receptors agonist, 1S,3R-ACPD did not alter the [3H]NECA binding observed in fresh non-washed-membranes. Therefore, the adenosine A2 receptor found in the optic tectum must be the adenosine A2b receptor which is available only in fresh membrane preparations, and its not modulated by guanine nucleotides or glutamate analogs.
Similar content being viewed by others
REFERENCES
Fredholm, B. B., Abbracchio, M. P., Burnstock, G., Daly, J. W., Harden, T. K., Jacobson, K. A, Leff, P., and Williams, M. 1994. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 46:143–156.
Ribeiro, J. A., and Sebastião, A. M. 1991. Purinergic modulation of neurotransmitter release in the peripheral and central nervous systems. In: Feigenbaun J, and Hanani M, eds. Presynaptic regulation of neurotransmitter release: a handbook. London: Freund Publishing House, p. 451–495.
Zhou, Q., Li, C., Olah, M. E., Johnson, R. A., and Stiles, G. L. 1992. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 89:7432–7436.
Latini, S., Pazzgli, M., Pepeu, G., and Pedata, F. 1996. Adenosine A2 receptors: their presence and neuromodulatory role in the central nervous system. Gen. Pharmacol. 27:925–933.
Jarvis, M. F., and Williams, M. 1989. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur. J. Pharmacol. 168:243–246.
Cunha, R. A, Johanson, B., van der Ploeg, I., Sebastião, A. M., Ribeiro, J. A., and Fredholm, B. B. 1994. The binding sites of A2a adenosine receptor agonist [3H]CGS 21680 are different in the hippocampus and cerebral cortex and in the striatum of the rat. Drug Develop. Res. 31:260.
Sebastião, A. M., and Ribeiro, J. A. 1996. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Progress in Neurobiol. 48:167–189.
Bruns, R. F., Lu, G. H., Pugsley, T. A. 1986. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Molec. Pharmacol. 29:331–346.
Ventura, A. L. M., and Paes de Carvalho, R. 1987. Development of adenosine-dependent cyclic AMP accumulation in the avian optic tectum. Devl. Brain Res. 35:141–147.
Tasca, C. I., Vendite, D., Martini, L. H., Cardoso, L. F., and Souza, D. O. 1995. Modulation of adenosine-induced cAMP accumulation via metabotropic glutamate receptors in chick optic tectum. Neurochem. Res. 20:1033–1039.
Holmann, M., and Heinemann, S. 1994. Cloned glutamate receptors. Annu. Rev. Neurosci. 17:31–108.
Hepler, J. R., and Gilman, A. G. 1992. G Proteins. Trends Biochem. Sci. 17:383–387.
Gudermann, T., Schoneberg, T., and Schultz, G. 1997. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu. Rev. Neurosci. 20:399–427.
Monahan, J. B., Hood, W. F., Michel, J., and Compton, R. P. 1988. Effects of guanine nucleotides on N-methyl-D-aspartate receptor-ligand interactions. Mol. Pharmacol. 34:111–116.
Souza, D. O., and Ramírez, G. 1991. Effects of guanine nucleotides on kainic acid binding and on adenylate cyclase in chick optic tectum and cerebellum. J. Mol. Neurosci. 3:39–45.
Gorodinsky, A., Paas, Y., and Teichberg, V. I. 1993. A ligand binding study of the interactions of guanine nucleotides with non-NMDA receptors. Neurochem. Int. 23:285–291.
Paz, M. M., Ramos, M., Ramírez, G., and Souza, D. O. 1994. Differential effects of guanine nucleotides on kainic acid binding and on adenylate cyclase activity in chick optic tectum. FEBS Lett. 355:205–208.
Paas, Y., Devillers-Thiery, A., Changeux, J.-P., Medevielle, F., and Teichberg, V. I. 1996. Identification of an extracellular motif involved in the binding of guanine nucleotides by a glutamate receptor. EMBO J. 15:1548–1556.
Rubin, M. A., Medeiros, A. C., Rocha, P. C. B., Livi, C. B., Ramirez, G., and Souza, D. O. 1997. Effect of guanine nucleotides on [3H]Glutamate binding and on adenylate cyclase activity in rat brain membranes. Neurochem. Res. 22:181–187.
Tasca, C. I., Wofchuk, S. T., Souza, D. O., Ramírez, G., and Rodnight, R. 1995. Guanine nucleotides inhibit the stimulation of GFAP phosphorylation by glutamate. NeuroReport 6:249–252.
Tasca, C. I., Cardoso, L. F., Martini, L. H., Ramírez, G., and Souza, D. O. 1998. Guanine nucleotides inhibit cAMP accumulation induced by metabotropic glutamate receptors activation. Neurochem. Res. 23:183–188.
Malcon, C., Achaval, M., Komlus, S., Partata, W., Sauressig, M., Ramírez, G., and Souza, D. O. 1997. GMP protects against quinolinic acid-induced loss of NADPH diaphorase-positive cells in the rat striatum. Neurosci. Lett. 225:145–148.
Perla, A. S., Maraschin, J. F., and Souza, D. O. 1997. GMP and GDP inhibit seizures induced by quinolinic acid in mice. J. Neurological Sci., Suppl.: Abstract S200(3-43-06).
Tasca, C. I., Cardoso, L. F., Ramírez, G., and Souza, D. O. 1999. Effects of guanine nucleotides on adenosine and glutamate modulation of cAMP levels in optic tectum slices from chicks. Neurochem. Int. 34:213–220.
Tasca, C. I., Vendite, D., Garcia, K. L., and Souza, D. O. 1995. Effects of adenosine on cAMP production during early development in the optic tectum of chicks. Int. J. Devl. Neurosci. 13:545–553.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Jarvis, M. F., Schulz, R., Hutchinson, A. J., Do, H. U., Sills, M. A., and Williams, M. 1989. 3H-CGS 21680, a selective A2 adenosine receptor agonist, directly labels A2 receptors in rat brain. J. Pharmacol. Exp. Ther. 251:888–893.
Wan, W., Sutherland, G. R., and Geiger, J. D. 1990. Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human and rat brain: evidence for multiple affinity sites. J. Neurochem. 55:1763–1771.
Diocee, B. K., and Souness, J. E. 1987. Characterization of 5′-Nethyl-carboxamide [3H]adenosine binding to pig aorta smooth muscle membranes. Biochem. Pharmacol. 36:3621–3627.
Lohse, M. J., Elger, B., Lindenborn-Fotinos, J., Klotz, K. N., and Schwabe, U. 1988. Separation of solubilized A2 adenosine receptors of human platelets from non-receptor [3H]NECA binding sites by gel filtration. Naunyn-Schmiedeberg's Arch. Pharmacol. 337:64–68.
Keen, M., Kelly, E., Nobbs, P., and MacDermot, J. 1989. A selective binding site for 3H-NECA that is not an adenosine A2 receptor. Biochem. Pharmacol. 38:3827–3833.
Johansson, B., Ahlberg, S., van der Ploeg, I., Brené, S., Lindefors, N., Persson, H., and Fredholm, B. B. 1992. Effects of mono-and divalent ions on the binding of the adenosine analog CGS 21680 in rat striatum. Biochem. Pharmacol. 44:2365–2370.
Parkinson, F. E., and Fredholm, B. B. 1990. Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn-Schimiedeberg's Arch. Pharmacol. 342:85–89.
Fredholm, B. B., Linsdtrom, K., Dionisotti, S., and Ongini, E. 1998. [3H]SCH 58261, a selective adenosine A2A receptor antagonist, is a useful ligand in autoradiographic studies. J. Neurochem. 70:1210–1216.
Ramos, M., Souza, D. O., and Ramirez, G. 1997. Specific binding of [3H]GppNHp to extracellular membrane receptors in chick cerebellum: possible involvement of kainic acid receptors. FEBS Lett. 406:114–118.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tasca, C.I., Cardoso, L.F., Vendite, D. et al. Study of Adenosine A2 Receptors in Membrane Preparations from Optic Tectum of Chicks. Neurochem Res 24, 1067–1074 (1999). https://doi.org/10.1023/A:1021017112717
Issue Date:
DOI: https://doi.org/10.1023/A:1021017112717