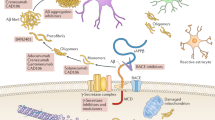
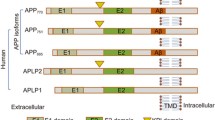
Abstract
1. Despite major efforts aimed at elucidating the molecular basis and physiopathology of Alzheimer's disease (AD), there is still no effective treatment available for this devastating disorder. The biological mechanisms underlying the development of AD are complex, as multiple factors appear to modulate (either positively or negatively) the progression of neurodegeneration in the brains of AD patients. Not surprisingly, a number of different therapeutic approaches aimed at distinct aspects of the disease are currently being pursued. Given its central role in the neuropathology of AD, the β-amyloid peptide (Aβ) is the focus of many such approaches.
2. In this review, we discuss recent developments along three major lines of investigation: (i) identification and characterization of inhibitors of the enzymes involved in proteolytic processing of the amyloid precursor protein and production of Aβ (ii) identification of the pathways involved in cerebral degradation and clearance of Aβ and (iii) characterization of small-molecule inhibitors of amyloid aggregation that prevent cerebral amyloid deposition and neurotoxicity.
3. Significant progress has been achieved in these directions, opening up new perspectives toward the development of effective approaches for the treatment or prevention of AD.
Similar content being viewed by others
REFERENCES
Abraham, C. R., McGraw, W. T., Slot, F., and Yamin, R. (2000). Alpha 1-antichymotrypsin inhibitsAbeta degradation in vitro and in vivo. Ann. N.Y. Acad. Sci. 920:245–248.
Arispe, N., Pollard, H. B., and Rojas, E. (1993a). Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc. Natl. Acad. Sci. U.S.A. 90:10573–10577.
Arispe, N., Pollard, H. B., and Rojas, E. (1996). Zn2C interaction with Alzheimer amyloid beta protein calcium channels. Proc. Natl. Acad. Sci. U.S.A. 93:1710–1715.
Arispe, N., Rojas, E., and Pollard, H. B. (1993b). Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. U.S.A. 90:567–571.
Asami-Odaka, A., Ishibashi, Y., Kikuchi, T., Kitada, C., and Suzuki, N. (1995). Long amyloid betaprotein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry 34:10272–10278.
Bacskai, B. J., Kajdasz, S. T., Christie, R. H., Carter, C., Games, D., Seubert, P., Schenk, D., and Hyman, B. T. (2001). Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 7(3):369–372.
Bai, D. L., Tang, X. C., and He, X. C. (2000). Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease. Curr. Med. Chem. 7:355–374.
Bard, F., Cannon, C., Barbour, R., Burke, R. L., Games, D., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Lieberburg, I., Motter, R., Nguyen, M., Soriano, F., Vasquez, N., Weiss, K., Welch, B., Seubert, P., Schenk,D., and Yednock, T. (2000). Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6:916–919.
Bhatia, R., Lin, H., and Lal, R. (2000). Zn2C interaction with Alzheimer amyloid beta protein calcium channels. FASEB J. 14:1233–1243.
Birmingham, K., and Frantz, S. (2002). Set back to Alzheimer vaccine studies. Nat. Med. 8:199–200.
Bodovitz, S., and Klein, W. L. (1996). Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 271:4435–4440.
Butterfield, D. A. (1997). beta-Amyloid-associated free radical oxidative stress and neurotoxicity: Implications for Alzheimer's disease. Chem. Res. Toxicol. 10:495–506.
Camilleri, P., Haskins, N. J., and Howlett, D. R. (1994). beta-Cyclodextrin interacts with the Alzheimer amyloid beta-A4 peptide. FEBS Lett. 341:256–258.
Chesneau,V., Vekrellis, K., Rosner, M. R., and Selkoe, D. J. (2000). Purified recombinant insulin-degrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem. J. 351:509–516.
Citron, M. (2000). Secretases as targets for the treatment of Alzheimer's disease. Mol. Med. Today 6:392–397.
Citron, M., Diehl, T. S., Capell, A., Haass, C., Teplow, D. B., and Selkoe, D. J. (1996). Inhibition of amyloid beta-protein production in neural cells by the serine protease inhibitor AEBSF. Neuron 17:171–179.
Cordell, G. A. (2000). Biodiversity and drug discovery—A symbiotic relationship. Phytochemistry 55:463–480.
Cragg, G. M., Newman, D. J., and Snader, K. M. (1997). Natural products in drug discovery and development. J. Nat. Prod. 60:52–60.
Czech, C., Tremp, G., and Pradier, L. (2000). Presenilins and Alzheimer's disease: Biological functions and pathogenic mechanisms.Prog. Neurobiol. 60:363–384.
da Cruz e Silva, E. F., da Cruz e Silva, A. O., Zaia, C. T., and Greengard, P. (1995). Inhibition of protein phosphatase 1 stimulates secretion of Alzheimer amyloid precursor protein. Mol. Med. 1:535–541.
De Felice, F. G., Houzel, J. C., Garcia-Abreu, J., Louzada, P. R., Jr., Afonso, R. C., Meirelles, M. N., Lent, R., Neto, V. M., and Ferreira, S. T. (2001). Inhibition of Alzheimer's disease beta-amyloid aggregation, neurotoxicity, and in vivo deposition by nitrophenols: Implications for Alzheimer's therapy. FASEB J. 15:1297–1929.
De Felice, F. G., and Ferreira, S. T. (2002). Physiopathological modulators of amyloid aggregation and novel pharmacological approaches in Alzheimer's disease. An. Acad. Bras. Cienc. 74:265–284.
Dovey, H.F., John,V., Anderson, J.P., Chen, L. Z., de Saint Andrieu, P., Fang, L.Y., Freedman, S.B., Folmer, B., Goldbach, E., Holsztynska, E. J., Hu, K. L., Johnson-Wood, K. L., Kennedy, S. L., Kholodenko,D., Knops, J. E., Latimer, L. H., Lee, M., Liao, Z., Lieberburg, I. M., Motter, R. N., Mutter, L. C., Nietz, J., Quinn, K. P., Sacchi, K. L., Seubert, P. A., Shopp, G. M., Thorsett, E. D., Tung, J. S., Wu, J., Yang, S., Yin, C. T., Schenk, D. B., May, P. C., Altstiel, L. D., Bender, M. H., Boggs, L. N., Britton, T. C., Clemens, J. C., Czilli, D. L., Dieckman-McGinty, D. K., Droste, J. J., Fuson, K. S., Gitter, B.D., Hyslop, P. A., Johnstone, E. M., Li, W. Y., Little, S. P., Mabry, T. E., Miller, F. D., and Audia, J. E. (2001). Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 76:173–181.
Edbauer, D., Winkler, E., Haass, C., and Steiner, H. (2002). Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc. Natl. Acad. Sci. U.S.A. 99:8666–8671.
Edgar, P. F., Douglas, J. E., Cooper, G. J., Dean, B., Kydd, R., and Faull, R. L. (2000). Comparative proteome analysis of the hippocampus implicates chromosome 6q in schizophrenia. Mol. Psychiatry 5:85–90.
Eikelenboom, P., Zhan, S. S., van Gool, W. A., and Allsop, D. (1994). Inflammatory mechanisms in Alzheimer's disease. Trends Pharmacol. Sci. 15:447–450.
Esler, W. P., and Wolfe, M. S. (2001).Aportrait of Alzheimer secretases—New features and familiar faces. Science 293:1449–1454.
Fassbender, K., Simons, M., Bergmann, C., Stroick, M., Lutjohann, D., Keller, P., Runz, H., Kuhl, S., Bertsch, T., von Bergmann, K., Hennerici, M., Beyreuther, K., and Hartmann, T. (2001). Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 98:5856–5861.
Ferreira, S. T., and De Felice, F. G. (2001). Protein dynamics, folding and misfolding: From basic physical chemistry to human conformational diseases. FEBS Lett. 498:129–134.
Gandy, S., and Greengard, P. (1994). Processing of Alzheimer A beta-amyloid precursor protein: Cell biology, regulation, and role in Alzheimer disease. Int. Rev. Neurobiol. 36:29–50.
Gasparini, L., Gouras, G. K., Wang, R., Gross, R. S., Beal, M. F., Greengard, P., and Xu, H. (2001). Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 21:2561–2570.
Geula, C., Wu, C. K., Saroff, D., Lorenzo, A., Yuan, M., and Yankner, B. A. (1998). Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat. Med. 4:827–831.
Ghanta, J., Shen, C. L., Kiessling, L. L., and Murphy, R. M. (1996). A strategy for designing inhibitors of beta-amyloid toxicity. J. Biol. Chem. 271:19525–19528.
Giacobini, E. (1997). From molecular structure to Alzheimer therapy. Jpn. J. Pharmacol. 74:225–241.
Glenner, G. G., and Wong, C.W. (1984). Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120:885–890.
Golde, T. E., and Eckman, C. B. (2001). Cholesterol modulation as an emerging strategy for the treatment of Alzheimer's disease. Drug. Discov. Today 6:1049–1055.
Grant, S. G., and Blackstock, W. P. (2001). Proteomics in neuroscience: From protein to network. J. Neurosci. 21:8315–8318.
Hardy, J. (1997). The Alzheimer family of diseases: Many etiologies, one pathogenesis? Proc. Natl. Acad. Sci. U.S.A. 94:2095–2097.
Haugabook, S. J., Yager, D. M., Eckman, E. A., Golde, T. E., Younkin, S.G., and Eckman, C.B. (2001). High throughput screens for the identification of compounds that alter the accumulation of the Alzheimer's amyloid beta peptide (Abeta). J. Neurosci. Methods 108:171–179.
Higaki, J., Quon, D., Zhong, Z., and Cordell, B. (1995). Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron 14:651–659.
Hong, L.,Koelsch,G., Lin, X., Wu, S., Terzyan, S., Ghosh, A. K., Zhang, X.C., and Tang, J. (2000). Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290:150–153.
Howlett, D. R., George, A. R., Owen, D. E., Ward, R.V., and Markwell, R. E. (1999a). Common structural features determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitors of Alzheimer beta-amyloid fibril formation. Biochem. J. 343:419–423.
Howlett, D. R., Perry, A. E., Godfrey, F., Swatton, J. E., Jennings, K. H., Spitzfaden, C., Wadsworth, H., Wood, S. J., and Markwell, R. E. (1999b). Inhibition of fibril formation in beta-amyloid peptide by a novel series of benzofurans. Biochem. J. 340:283–289.
Hu, J., Igarashi, A., Kamata, M., and Nakagawa, H. (2001). Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta ); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J. Biol. Chem. 276:47863–47868.
Hughes, E., Burke, R. M., and Doig, A. J. (2000). Inhibition of toxicity in the beta-amyloid peptide fragment beta-(25-35) using N-methylated derivatives: A general strategy to prevent amyloid formation. J. Biol. Chem. 275:25109–25115.
Iwata, N., Tsubuki, S., Takaki, Y., Shirotani, K., Lu, B., Gerard, N. P., Gerard, C., Hama, E., Lee, H. J., and Saido, T. C. (2001). Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552.
Iwata, N., Tsubuki, S., Takaki, Y., Watanabe, K., Sekiguchi, M., Hosoki, E., Kawashima-Morishima, M., Lee, H. J., Hama, E., Sekine-Aizawa, Y., and Saido, T.C. (2000). Identification of the major Abeta 1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 6:143–150.
Iwatsubo, T., Odaka, A., Suzuki, N., Mizusawa, H., Nukina, N., and Ihara, Y. (1994). Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: Evidence that an initially deposited species is A beta 42(43). Neuron 3:45–53.
Janus, C., Pearson, J., McLaurin, J., Mathews, P. M., Jiang, Y., Schmidt, S. D., Chishti, M. A., Horne, P., Heslin,D., French, J., Mount, H. T., Nixon, R. A., Mercken, M., Bergeron, C., Fraser, P. E., St George-Hyslop, P., and Westaway, D. (2000). A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408:979–982.
Jarrett, J. T., Berger, E. P., and Lansbury, P. T., Jr. (1993). The C-terminus of the beta protein is critical in amyloidogenesis. Ann. N.Y. Acad. Sci. 695:144–148.
Jick, H., Zornberg, G. L., Jick, S. S., Seshadri, S., and Drachman, D. A. (2000). Statins and the risk of dementia. Lancet 356:1627–1631.
Kakimura, J., Kitamura, Y., Taniguchi, T., Shimohama, S., and Gebicke-Haerter, P. J. (2001). Bip/GRP78-induced production of cytokines and uptake of amyloid-beta(1-42) peptide in microglia. Biochem. Biophys. Res. Commun. 281:6–10.
Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K., and Muller-Hill, B. (1987). The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–736.
Kimberly, W. T., LaVoie, M. J., Ostaszewski, B. L., Ye,W., Wolfe, M. S., and Selkoe, D. J. (2002). Complex N-linked glycosylated Nicastrin associates with active gamma-secretase and undergoes tight cellular regulation. J. Biol. Chem. 277:35113–35117
Klunk, W. E., Debnath, M. L., Koros, A. M., and Pettegrew, J. W. (1998). Chrysamine-G, a lipophilic analogue of Congo red, inhibits A beta-induced toxicity in PC12 cells. Life Sci. 63:1807–1814.
Knops, J., Suomensaari, S., Lee, M., McConlogue, L., Seubert, P., and Sinha, S. (1995). Cell-type and amyloid precursor protein-type specific inhibition of A beta release by bafilomycin A1, a selective inhibitor of vacuolar ATPases. J. Biol. Chem. 270:2419–2422.
Lauer, D., Reichenbach, A., and Birkenmeier, G. (2001). Alpha 2-macroglobulin-mediated degradation of amyloid beta 1–42: A mechanism to enhance amyloid beta catabolism. Exp. Neurol. 167:385–392.
Li, M. D., Kane, J. K., Matta, S. G., Blaner, W. S., and Sharp, B. M. (2000). Nicotine enhances the biosynthesis and secretion of transthyretin from the choroid plexus in rats: Implications for beta-amyloid formation. J. Neurosci. 20:1318–1823.
Lin, X., Koelsch, G., Wu, S., Downs, D., Dashti, A., and Tang, J. (2000). Proc. Natl. Acad. Sci. U.S.A. 97:1456.
Lin, Y. M., Raffen, R., Zhou, Y., Cassidy, C. S., Flavin, M. T., and Stevens, F. J. (2001). Amyloid fibril formation in microwell plates for screening of inhibitors. Amyloid 8:182–193.
Lorenzo, A., and Yankner, B. A. (1994). Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. U.S.A. 91:12243–12247.
Louzada, P. R., Jr., Paula Lima, A. C., de Mello, F.G., and Ferreira, S. T. (2001). Dual role of glutamatergic neurotransmission on amyloid beta(1-42) aggregation and neurotoxicity in embryonic avian retina. Neurosci. Lett. 301:59–63.
Lowe, T. L., Strzelec, A., Kiessling, L. L., and Murphy, R. M. (2001). Structure–function relationships for inhibitors of beta-amyloid toxicity containing the recognition sequence KLVFF. Biochemistry 40:7882–7889.
Luo, Y., Bolon, B., Kahn, S., Bennett, B. D., Babu-Khan, S., Denis, P., Fan, W., Kha, H., Zhang, J., Gong, Y., Martin, L., Louis, J. C., Yan, Q., Richards, W. G., Citron, M., and Vassar, R. (2001). Nat. Neurosci. 4:231.
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., and Beyreuther, K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci.U.S.A. 82:4245–4529.
Mattson, M. P. (1992). Calcium as sculptor and destroyer of neural circuitry. Exp. Gerontol. 27:29–49.
Mattson, M. P., Cheng,B., Davis,D., Bryant, K., Lieberburg, I., and Rydel R. E. (1992). beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12: 376–389.
Mirnics, K., Middleton, F. A., Lewis, D. A., and Levitt, P. (2001). Analysis of complex brain disorders with gene expression microarrays: Schizophrenia as a disease of the synapse. Trends. Neurosci. 24:479–486.
Mirnics, K., Middleton, F. A., Marquez, A., Lewis, D. A., and Levitt, P. (2000). Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 28:53–67.
Mohajeri, M. H., Wollmer, M. A., and Nitsch, R. M. (2002). Abeta 42-induced increase in neprilysin is associated with prevention of amyloid plaque formation in vivo. J. Biol. Chem. 277:35460–35465.
Moore, C. L., Diehl, T. S., Selkoe, D. J., and Wolfe, M. S. (2000). Toward the characterization and identification of gamma-secretases using transition-state analogue inhibitors. Ann.N.Y. Acad. Sci. 920:197–205.
Morgan, D., Diamond, D. M., Gottschall, P. E., Ugen, K. E., Dickey, C., Hardy, J., Duff, K., Jantzen, P., DiCarlo,G., Wilcock,D., Connor, K., Hatcher, J., Hope, C., Gordon, M., and Arendash, G.W. (2000). A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408(6815):982–985.
Mucke, L., Yu, G. Q., McConlogue, L., Rockenstein, E. M., Abraham, C. R., and Masliah, E. (2000). Astroglial expression of human alpha(1)-antichymotrypsin enhances Alzheimerlike pathology in amyloid protein precursor transgenic mice. Am. J. Pathol. 157:2003–2010.
Mukherjee, A., Song, E., Kihiko-Ehmann, M., Goodman, J. P., Jr., Pyrek, J. S., Estus, S., and Hersh, L. B. (2000). Insulysin hydrolyzes amyloid beta peptides to products that are neither neurotoxic nor deposit on amyloid plaques. J. Neurosci. 20:8745–8749.
National Institute on Aging. (1998). Progress report on Alzheimer's disease, National Institute on Aging Bethesda, MD, USA.
Nunan, J., and Small, D. H. (2000). Regulation of APP cleavage by alpha-, beta-and gamma-secretases. FEBS Lett. 483:6–10.
Oken, B. S., Storzbach, D. M., and Kaye, J. A. (1998). The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch. Neurol. 55:1409–1415.
Pallitto, M. M., Ghanta, J., Heinzelman, P., Kiessling, L. L., and Murphy, R. M. (1999). Recognition sequence design for peptidyl modulators of beta-amyloid aggregation and toxicity. Biochemistry 38:3570–3578.
Pappolla, M., Bozner, P., Soto, C., Shao, H., Robakis, N. K., Zagorski, M., Frangione, B., and Ghiso J. (1998). Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J. Biol. Chem. 273:7185–7188.
Pappolla, M. A., Sos, M., Omar, R. A., Bick, R. J., Hickson-Bick, D. L., Reiter, R. J., Efthimiopoulos, S., and Robakis, N. K. (1997). Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 17:1683–1690.
Paresce, D. M., Ghosh, R. N., and Maxfield, F. R. (1996). Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron 17:553–565.
Pepys, M. B., Herbert, J., Hutchinson, W. L., Tennent, G. A., Lachmann, H. J., Gallimore, J. R., Lovat, L. B., Bartfai, T., Alanine, A., Hertel, C., Hoffmann, T., Jakob-Roetne, R., Norcross, R. D., Kemp, J. A., Yamamura, K., Suzuki, M., Taylor, G.W., Murray, S., Thompson,D., Purvis, A., Kolstoe, S., Wood, S. P., and Hawkins, P. N. (2002). Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417:254–259.
Pike, C. J., Burdick, D., Walencewicz, A. J., Glabe, C. G.,and Cotman, C. W. (1993). Neurodegeneration induced by beta-amyloid peptides in vitro: The role of peptide assembly state. J. Neurosci. 13:1676–1687.
Poduslo, J. F., Curran, G. L., Kumar, A., Frangione, B., and Soto, C. (1999). Beta-sheet breaker peptide inhibitor of Alzheimer's amyloidogenesis with increased blood–brain barrier permeability and resistance to proteolytic degradation in plasma. J. Neurobiol. 39:371–382.
Poeggeler, B., Miravalle, L., Zagorski, M. G., Wisniewski, T., Chyan, Y. J., Zhang, Y., Shao, H., Bryant-Thomas, T., Vidal, R., Frangione, B., Ghiso, J., and Pappolla, M. A. (2001). Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry 40:14995–5001.
Pollack, S. J., Sadler, I. I., Hawtin, S. R., Tailor, V. J., and Shearman, M. S. (1995). Sulfated glycosaminoglycans and dyes attenuate the neurotoxic effects of beta-amyloid in rat PC12 cells. Neurosci. Lett. 184:113–116.
Qiu, Z., Strickland, D. K., Hyman, B. T., and Rebeck, G. W. (1999). Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J. Neurochem. 73:1393–1398.
Raghu, P., Reddy, G. B., and Sivakumar, B. (2002). Inhibition of transthyretin amyloid fibril formation by 2,4-dinitrophenol through tetramer stabilization. Arch. Biochem. Biophys. 400:43–47.
Reixach, N., Crooks, E., Ostresh, J. M., Houghten, R. A., and Blondelle, S. E. (2000). Inhibition of betaamyloid-induced neurotoxicity by imidazopyridoindoles derived from a synthetic combinatorial library. J. Struct. Biol. 130:247–258.
Rogers, J., and Shen, Y. (2000). A perspective on inflammation in Alzheimer's disease. Ann. N.Y. Acad. Sci. 924:132–135.
Salomon, A. R., Marcinowski, K. J., Friedland, R. P., and Zagorski, M.G. (1996). Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry 35:13568–13578.
Schenk,D., Barbour, R., Dunn,W., Gordon,G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Liao, Z., Lieberburg, I., Motter, R., Mutter, L., Soriano, F., Shopp,G., Vasquez,N., Vandevert,C., Walker, S., Wogulis, M., Yednock,T., Games,D., and Seubert, P. (1999). Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177.
Scheuner, D., Eckman, C., Jensen, M., Song, X., Citron, M., Suzuki, N., Bird, T. D., Hardy, J., Hutton, M., Kukull, W., Larson, E., Levy-Lahad, E., Viitanen, M., Peskind, E., Poorkaj, P., Schellenberg, G., Tanzi, R., Wasco,W., Lannfelt, L., Selkoe, D., and Younkin, S. (1996). Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2:864–870.
Seiffert, D., Bradley, J. D., Rominger, C. M., Rominger, D. H., Yang, F., Meredith, J. E., Jr., Wang, Q., Roach, A. H., Thompson, L. A., Spitz, S. M., Higaki, J. N., Prakash, S. R., Combs, A. P., Copeland, R. A., Arneric, S. P., Hartig, P. R., Robertson, D. W., Cordell, B., Stern, A. M., Olson, R. E., and Zaczek, R. (2000). Presenilin-1 and-2 are molecular targets for gamma-secretase inhibitors. J. Biol. Chem. 275:34086–34091.
Selkoe, D. J. (1991).The molecular pathology of Alzheimer's disease. Neuron 6:487–498.
Selkoe, D. J. (1994). Amyloid beta-protein precursor: New clues to the genesis of Alzheimer's disease. Curr. Opin. Neurobiol. 4:708–716.
Selkoe, D. J. (1996). Amyloid beta-protein and the genetics of Alzheimer's disease. J. Biol. Chem. 271:18295–18298.
Selkoe, D. J. (1999). Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399:A23–A31.
Sennrik, K., Benedikz, E., Fastbow, J., Sundstrom, E., Winblad, B., and Aukarcroma, M. (2001) Calcium ionophore A23187 specifically decreases the secretion of beta-secretase cleaved amyloid precursor protein during apoptosis in primary rat cortical cultures. J. Neurosc. Res. 63:429–437.
Shaffer, L. M., Dority, M. D., Gupta-Bansal, R., Frederickson, R. C., Younkin, S. G., and Brunden, K. R. (1995). Amyloid beta protein (A beta) removal by neuroglial cells in culture. Neurobiol. Aging 16:737–745.
Shirotani, K., Tsubuki, S., Iwata, N., Takaki, Y., Harigaya, W., Maruyama, K., Kiryu-Seo, S., Kiyama, H., Iwata, H., Tomita, T., Iwatsubo, T., and Saido, T. C. (2001). Neprilysin degrades both amyloid beta peptides 1-40 and 1-42 most rapidly and efficiently among thiorphan-and phosphoramidon-sensitive endopeptidases. J. Biol. Chem. 276:21895–21901.
Sigurdsson, E. M., Permanne, B., Soto, C., Wisniewski, T., and Frangione, B. (2000). In vivo reversal of amyloid-beta lesions in rat brain. J. Neuropathol. Exp. Neurol. 59:11–17.
Sinha, S., Anderson, J. P., Barbour, R., Basi, G. S., Caccavello, R., Davis, D., Doan, M., Dovey, H. F., Frigon, N., Hong, J., Jacobson-Croak, K., Jewett, N., Keim, P., Knops, J., Lieberburg, I., Power, M., Tan, H., Tatsuno, G., Tung, J., Schenk, D., Seubert, P., Suomensaari, S. M., Wang, S., Walker, D., and John, V. (1999). Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402:537–540.
Small, D. H., and McLean, C. A. (1999). Alzheimer's disease and the amyloid beta protein: What is the role of amyloid? J. Neurochem. 73:443–449.
Soto, C., Kascsak, R. J., Saborio, G. P., Aucouturier, P., Wisniewski, T., Prelli, F., Kascsak, R., Mendez, E., Harris, D. A., Ironside, J., Tagliavini, F., Carp, R. I., and Frangione, B. (2000). Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet 355:192–197.
Soto, C., Kindy, M. S., Baumann, M., and Frangione, B. (1996). Inhibition of Alzheimer's amyloidosis by peptides that prevent beta-sheet conformation. Biochem. Biophys. Res. Commun. 226:672–680.
Soto, C., Sigurdsson, E. M., Morelli, L., Kumar, R. A., Castano, E. M., and Frangione, B. (1998). Betasheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for Alzheimer's therapy. Nat. Med. 4:822–826.
Steinhilb, M. L., Turner, R. S., and Gaut, J. R. (2001). The protease inhibitor, MG132, blocks maturation of the amyloid precursor protein Swedish mutant preventing cleavage by beta-Secretase. J. Biol. Chem. 276:4476–4484.
St. George-Hyslop, P. H., and Westaway, D.A. (1999). Alzheimer's disease. Antibody clears senile plaques. Nature 400:116–117.
Tomiyama, T., Shoji, A., Kataoka, K., Suwa, Y., Asano, S., Kaneko, H., and Endo, N. (1996). Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J. Biol. Chem. 271:6839–6844.
Tsuzuki, K., Fukatsu, R., Yamaguchi, H., Tateno, M., Imai, K., Fujii, N., and Yamauchi, T. (2000). Transthyretin binds amyloid beta peptides, Abeta 1-42 and Abeta 1-40 to form complex in the autopsied human kidney—Possible role of transthyretin for abeta sequestration. Neurosci. Lett. 281:171–174.
Varadarajan, S., Yatin, S., Aksenova, M., and Butterfield, D. A. (2000). Alzheimer's amyloid beta-peptideassociated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 130:184–208.
Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., Teplow, D. B., Ross, S., Amarante, P., Loeloff, R., Luo, Y., Fisher, S., Fuller, J., Edenson, S., Lile, J., Jarosinski, M. A., Biere, A. L., Curran, E., Burgess, T., Louis, J. C., Collins, F., Treanor, J., Rogers, G., and Citron, M. (1999). Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741.
Vekrellis, K., Ye, Z., Qiu, W.Q., Walsh,D., Hartley,D., Chesneau,V., Rosner, M. R., and Selkoe, D. J. (2000). Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 20:1657–1665.
Verbeek, M. M., Ruiter, D. J., and de Waal, R. M. (1997). The role of amyloid in the pathogenesis of Alzheimer's disease. Biol. Chem. 378: 937–950.
Wolozin, B. (2001).Afluid connection: Cholesterol and Abeta. Proc. Natl. Acad. Sci.U.S.A. 98:5371–5373.
Wolozin, B., Kellman, W., Ruosseau, P., Celesia, G. G., and Siegel, G. (2000). Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 57:1439–1443.
Wood, S. J., MacKenzie, L., Maleeff, B., Hurle, M. R., and Wetzel, R. (1996). Selective inhibition of Abeta fibril formation. J. Biol. Chem. 271:4086–4092.
Yan, R., Bienkowski, M. J., Shuck, M. E., Miao, H., Tory, M. C., Pauley, A. M., Brashier, J. R., Stratman, N. C., Mathews, W. R., Buhl, A. E., Carter, D. B., Tomasselli, A.G., Parodi, L. A., Heinrikson, R. L., and Gurney, M. E. (1999). Membrane-anchored aspartyl protease with Alzheimer's disease betasecretase activity. Nature 402:533–537.
Yu, G., Nishimura, M., Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., Supala, A., Levesque, L., Yu, H., Yang, D. S., Holmes, E., Milman, P., Liang, Y., Zhang, D. M., Xu, D. H., Sato, C., Rogaev, E., Smith, M., Janus, C., Zhang, Y., Aebersold, R., Farrer, L. S., Sorbi, S., Bruni, A., Fraser, P., and St. George-Hyslop, P. (2000). Nicastrin modulates presenilinmediated notch/glp-1 signal transduction and betaAPP processing. Nature 407:48–54.
Zamani, M. R., Allen, Y. S., Owen, G. P., and Gray, J. A. (1997). Nicotine modulates the neurotoxic effect of beta-amyloid protein(25-35) in hippocampal cultures. Neuroreport 8:513–517.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Felice, F.G., Ferreira, S.T. β-Amyloid Production, Aggregation, and Clearance as Targets for Therapy in Alzheimer's Disease. Cell Mol Neurobiol 22, 545–563 (2002). https://doi.org/10.1023/A:1021832302524
Issue Date:
DOI: https://doi.org/10.1023/A:1021832302524