Abstract
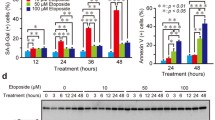
Alterations in gene expression during apoptosis in HL-60 cells were identified by a cDNA based array analysis. Apoptosis was induced in the human promyelocytic leukemia cell line, HL-60, by incubation with 30 μM etoposide for 5 hours. Changes in gene expression occurring during apoptosis in these cells were detected using the “ATLAS cDNA Expression Array” technique. 40 genes were identified as differentially expressed in the apoptotic cells by at least a factor of two. 30 of these genes were down-regulated during apoptosis. Many of the down-regulated genes reflected decreased proliferative activity in the cells as well as decreased activity of survival pathways. Most of the genes, which were up-regulated during apoptosis, were genes involved in pathways leading to cell death and suppression of proliferation. Based on the up-regulations observed at the mRNA level, it is speculated that etoposide-induced apoptosis in the HL-60 cells proceeds via pathways involving factors such as TNFα, IGFBP3, SAPK1, AP-1 and GADD153/CHOP10. Four genes, which showed changes at the mRNA level, were also analyzed by Western blotting in order to confirm the observed differences at the protein level.
Similar content being viewed by others
References
Solary E, Bertrand R, Pommier Y. Apoptosis induced by DNA topoisomerase I and II inhibitors in human leukemic HL-60 cells. Leuk Lymphoma 1994; 15(1/2): 21-32.
Long BH, Musial ST, Brattain MG. Comparison of cytotoxicity and DNA breakage activity of congeners of podophylltoxin including VP16-213 and VM26: A quantitative structure-activity relationship. Biochemistry 1984; 23: 1183-1188.
Loike JD, Horwitz SB. Effect of VP-16-213 on the intracellular degradation of DNAin HeLa cells. Biochemistry 1976; 15: 5443-5448.
Dubrez L, Goldwasser F, Genne P, Pommier Y, Solary E. The role of cell cycle regulation and apoptosis triggering in determining the sensitivity of leukemic cells to topoisomerase I and II inhibitors. Leukemia 1995; 9: 1013-1024.
Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB 1996; 10: 587-597.
Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
Wyllie AH. Glucocorticoid induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980; 284: 555-556.
Li S, MacLachlan TK, De Luca A, Claudio PP, Condorelli G, Giordano A. The cdc2-related kinase, PISSLRE, is essential for cell growth and acts in G2 phase of the cell cycle. Cancer Res 1995; 55: 3992-3995.
Guan KL, Jenkins CW, Li Y, et al. Isolation and characterization of p19INK4d, a p16-related inhibitor specific to CDK6 and CDK4. Mol Biol Cell 1996; 7: 57-70.
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 1995; 130: 507-518.
Kinoshita M, Kumar S, Mizoguchi A, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes and Development 1997; 11: 1535-1547.
Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 1995; 81: 261-268.
Zhou BB, Li H, Yuan J, Kirschner MW. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc Natl Acad Sci USA 1998; 95: 6785-6790.
Sheng-Ong GL. The myb oncogene. Biochem Biophys Acta 1990; 1032: 39-52.
Wolff L. Myb-induced transformation. Crit Rev Oncog 1996; 7: 245-260.
Vrana JA, Decker RH, Johnson CR, Wang Z, Jarvis W, Richon VM. Induction of apoptosisin U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP, but independent on p53. Oncogene 1999; 18: 7016-7025.
Ryan KM, Birnie GD. Myc oncogenes: The enigmatic family. Biochem J 1996; 314: 713-721.
Hoffman B, Liebermann DA. The proto-oncogene c-myc and apoptosis. Oncogene 1998; 17: 3351-3357.
Werten S, Langen FW, van Schaik R, Timmers HT, Meisterernst M, van der Vliet PC. High-affinity DNA binding by the C-terminal domain of the transcriptional coactivator PC4 requires simultaneous interaction wih two opposing unpaired strands and results in helix destabilization. J Mol Biol 1998; 276: 367-377.
Kannan P, Tainsky MA. Coactivator PC4 mediates AP-2 transcriptional activity and suppresses ras-induced transformation dependt on AP-2 transcriptional interference. Mol Cell Biol 1999; 19: 899-908.
Okada T, Masuda T, Shinkai M, Kariya K, Kataoka T. Posttranslational modification of H-Ras is required for activation of, but not for association with, B-Raf. J Biol Chem 1996; 271: 4671-4678.
Lee SY, Lee SY, Choi Y. TRAF interacting protein (TRIP): A novel component of the tumor necrosis factor receptor (TNFR)-CD30-TRAF signaling complex that inhibits TRAF2 mediated NF-?B activation. J Exp Med 1997; 185: 1275-1286.
Kuhnel F, Zender L, Paul Y, et al. NfkappaB mediated apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem 2000; 275: 6421-6427.
Force WR, Cheung TC, Ware CF. Dominant negative mutants of TRAF3 reveal an important role for the coiled coil domains in cell death signalling by the lymphotoxin-beta receptor. J Biol Chem 1997; 272: 30835-30840.
Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406: 532-535.
Gomez-Marquez J, Rodriguez P. Prothymosin alpha is a chromatin-remodeling protein in mammalian cells. Biochem J 1998; 333: 1-3.
Smith MR. Prothymosin alpha: In search of a function. Leuk Lymphoma 1995; 18: 209-214.
Evstafieva AG, Belov GA, Kalkum M, et al. Prothymosin α fragmentation in apoptosis. FEBS Lett 2000; 467: 150-154.
Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of MCL-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol 1995; 128: 1173-1184.
Ohta K, Iwai K, Kasahara Y, et al. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and MCL-1, in human peripheral blood and lymphoid tissues. Int Immunol 1995; 7: 1817-1825.
Lee M, Han S, Jiang S, Park JH, Kim SJ. Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor ?. Biochem Pharmacol 2000; 59: 485-496.
Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: A new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat 1995; 35: 115-132.
Gilbertson RJ, Clifford SC, MacMeekin W, et al. Expression of the ErbB-neuregulin signaling network during human cerebellar development: Implications for the biology of medulloblastoma. Cancer Res 1998; 58: 3932-3941.
Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem 2000; 275: 39174-39181.
Abate C, Curran T. Encounters with Fos and Jun on the road to AP-1. Semin Cancer Biol 1990; 1: 19-26.
Sawai H, Okazaki T, Yamamoto H, et al. Requirement of AP-1 for ceramide-induced apoptosis in human leukemia HL-60 cells. J Biol Chem 1995; 270: 27326-27331.
Kondo T, Matsuda T, Kitano T, Takahashi A, Tashima M, Ishikura H. Role of c-jun expression increased by heat shockand ceramide-activated caspase-3 in HL-60 cell apoptosis. Possible involvement of ceramide in heat shock-induced apoptosis. J Biol Chem 2000; 275: 7668-7676.
Liebermann DA, Gregory B, Hoffmann B. AP-1 (Fos/Jun) transcription factors in hematopoietic differentiation and apoptosis. Int J Oncol 1998; 12: 685-700.
Chen YI, Tan TH. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int J Oncol 2000; 16: 651-662.
Derijard B, Hibi M, Wu IH, et al. JNK1:Aprotein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994; 76: 1025-1037.
Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. CHOP10 (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on induction of G1/S arrest. Genes Dev 1994; 8: 453-464.
Sylvester SL, ap Rhys CM, Luethy-Martindale JD, Holbrook NJ. Induction of GADD153, a CCAAT/enhancer-binding protein (C/EBP)-related gene, during the acute phase response in rats. Evidence for the involvement of C/EBPs in regulating its expression. J Biol Chem 1994; 269: 20119-20125.
Ubeda M, Wang XZ, Zinser H, Habener JF, Ron D. Stressinduced binding of the transcription factor CHOP to a novel DNA control element. Mol Cell Biol 1996; 16: 1479-1489.
Kim R, Ohi Y, Inoue H, Aogi K, Toge T. Introduction of gadd153 gene into gastric cancer cells can modulate sensitivity to anticancer agents in association with apoptosis. Anticancer Res 1999; 19: 1779-1783.
Kim R, Ohi Y, Inoue H, Toge T. Taxotere activates transcription factor AP-1 in association with apoptotic cell death in gastric cancer cell lines. Anticancer Res 1999; 19: 5399-5405.
Eymin B, Dubrez L, Allouche M, Solary E. Increased gadd153 messenger RNA level is associated with apoptosis in human leukemic cells treated with etoposide. Cancer Res 1997; 686-695.
van Antwerp DJ, Martin SJ, Verma IM, Green DR. Inhibition of TNF-induced apoptosis by NF-?B. Trends Cell Biol 1998; 8: 107-111.
Wallach D, Kovalenko, AV, Varfolomeev EE, Boldin MP. Death-inducing functions of ligands of the tumor necrosis factor family: A Sanhedrin verdict. Curr Opin Immunol 1998; 10: 279-288.
Oei SL, Griesenbeck J, Schweiger M, Ziegler M. Regulation of RNA polymerase II-dependent transcription by poly (ADPribosyl) ation of transcription factors. J Biol Chem 1998; 273: 31644-31647.
Duriez PJ, Shah GM. Cleavage of poly(ADP-ribose) polymerase: A sensitive parameter to study cell death. Biochem Cell Biol 1997; 75: 337-349.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bjørling-Poulsen, M., Issinger, OG. cDNA array analysis of alterations in gene expression in the promyelocytic leukemia cell line, HL-60, after apoptosis induction with etoposide. Apoptosis 8, 377–388 (2003). https://doi.org/10.1023/A:1024177119658
Issue Date:
DOI: https://doi.org/10.1023/A:1024177119658