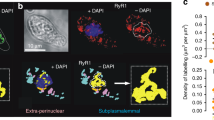
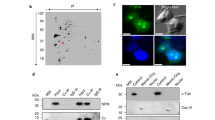
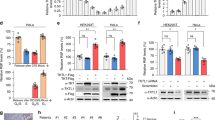
Abstract
Nucleoplasmic calcium ions (Ca2+) influence nuclear functions as critical as gene transcription, apoptosis, DNA repair, topoisomerase activation and polymerase unfolding. Although both inositol trisphosphate receptors and ryanodine receptors, types of Ca2+ channel, are present in the nuclear membrane, their role in the homeostasis of nuclear Ca2+ remains unclear. Here we report the existence in the inner nuclear membrane of a functionally active CD38/ADP-ribosyl cyclase that has its catalytic site within the nucleoplasm. We propose that the enzyme catalyses the intranuclear cyclization of nicotinamide adenine dinucleotide to cyclic adenosine diphosphate ribose. The latter activates ryanodine receptors of the inner nuclear membrane to trigger nucleoplasmic Ca2+ release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Malviya, A. N. & Rogue, P. J. ‘‘Tell me where is calcium bred’’: clarifying the roles of nuclear calcium. Cell 92, 17–23 ( 1998).
Santella, L. & Carafoli, E. Calcium signaling in the cell nucleus . FASEB J. 11, 1091–1109 (1997).
Al-Mohanna, F. A. et al. The nucleus is insulated from large cytosolic calcium ion changes. Nature 367, 745– 750 (1994).
Perez-Terzic, C., Stehno-Bittel, L. & Clapham, D. E. Nucleoplasmic and cytoplasmic differences in the fluorescent properties of calcium indicator fluo-3. Cell Calcium 21, 275–282 ( 1997).
Fox, J. L., Burgstahler, A. D. & Nathanson, M. H. Mechanism of long-range Ca2+ signaling in the nucleus of isolated rat hepatocytes. Biochem J. 326, 491–495 (1997).
Gerasimenko, O. V. et al. ATP-dependent accumulation and inositol triphosphate or cyclic ADP ribose-mediated release of calcium from the nuclear envelope. Cell 80, 439–444 ( 1995).
Hennager, D. J., Welsh, M. J. & DeLisle, S. Changes in either cytosolic or nuleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear calcium concentration. J. Biol. Chem. 270, 4959–4962 (1995).
Santella, L. & Kyozuka, K. Calcium release into the nucleus by 1,4,5-trisphosphate and cyclic ADP-ribose gated channels induced the resumption of meiosis in starfish oocytes. Cell Calcium 22, 1–10 (1997).
Mehta, M., Shahid, U. & Malavasi, F. Human CD38, a cell surface protein with multiple functions . FASEB J. 10, 1408–1417 (1996).
Zaidi, M. et al. A ryanodine receptor-like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. J. Clin. Invest. 96, 1582–1590 (1995).
Morita, K., Kitayama, S. & Dohi, T. Stimulation of cyclic ADP-ribose synthesis by acetylcholine and its role in catecholamine release in bovine adrenal chromaffin cells. J. Biol. Chem. 272, 21002–21009 (1997).
Anandathreerthvardha, H. K. et al. Localization of multiple forms of inducible cytochromes P 450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch. Biochem. Biophys. 339, 136–150 (1997).
Humbert, J. P. et al. Inositol 1,4,5,-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear Ca2+ signaling by inositol 1,4,5-trisphosphate. J. Biol. Chem. 271, 478–485 ( 1996).
Matter, N. & Malviya, A. N. Calcium transported to isolated rat liver nuclei by nicotinamide adenine dinucleotide is insensitive to thapsigargin . FEBS Lett. 387, 85–88 (1996).
Malviya, A. N., Rogue, P. & Vincendon, G. Sterospecific inositol 1,4,5-[32P] trisphosphate binding to isolated rat liver nuclei. Evidence for inositol trisphosphate receptor mediated calcium release from the nucleus. Proc. Natl Acad. Sci. USA. 87, 9270– 9274 (1990).
Nicotera, P. et al. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc. Natl Acad. Sci. USA. 87, 6858–6862 (1990).
Mak, D. O. D., & Foskett, J. K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 269, 29375–29378 (1994).
Capitani, S. et al. Uptake and phosphorylation of phosphatidyl inositol by rat liver nuclei: role of phosphatidyl inositol transfer protein. Biochim. Biophys. Acta. 1044, 193– 200 (1990).
Payraste, B. et al. A differential localization of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J. Biol. Chem. 267, 5078–5084 ( 1992).
Asano, M. et al. Purification and characterization of nuclear phospholipase C specific for phosphoinositides. J. Biol. Chem. 269, 12360–12366 (1994).
Hardingham, G. E. et al. Distinct functions of nuclear and cytoplasmic Ca2+ in the control of gene expression. Nature 285, 260 (1996).
Ginty, D. D., Bonni, A. & Greenberg, M. E. Nerve growth factor activates a ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77, 713–725 (1996).
Hill, C. S. & Treisman, R. Transcription regulation by extracellular signals: mechanisms and specificity. Cell 80, 199–211 (1995).
Larea, L. S. & McNamara, O. J. Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium-requiring intracellular signaling pathways. Neuron 10, 31–41 (1993).
Adebanjo, O. A. et al. Mode of action of interleukin-6 on mature osteoclasts. Novel interactions with extracellular Ca2+ sensing in the regulation of osteoclastic bone resorption. J. Cell Biol. 142, 1347–1356 (1998).
Chawla, S. et al. CBP: a signal-regulated transcriptional co-activator controlled by nuclear calcium and CaM kinase IV. Science 281, 1505–1509 (1998).
Biswas G. et al. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J 18, 522 –533 (1998).
Acknowledgements
This study was supported by grants from the NIH (RO1-AG14917, to M.Z., and R37-CA22762, to N.G.A.) and by the Department of Veterans Affairs (Merit Review to M.Z.). We thank I. MacIntyre and T. Davies for support.
Correspondence and requests for materials should be addressed to M.Z.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adebanjo, O., Anandatheerthavarada, H., Koval, A. et al. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol 1, 409–414 (1999). https://doi.org/10.1038/15640
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/15640
This article is cited by
-
CD38/ADP-ribose/TRPM2-mediated nuclear Ca2+ signaling is essential for hepatic gluconeogenesis in fasting and diabetes
Experimental & Molecular Medicine (2023)
-
Probing the requirement for CD38 in retinoic acid-induced HL-60 cell differentiation with a small molecule dimerizer and genetic knockout
Scientific Reports (2017)
-
CD38-mediated Ca2+ signaling contributes to glucagon-induced hepatic gluconeogenesis
Scientific Reports (2015)
-
The pathogenic relevance of the prognostic markers CD38 and CD49d in chronic lymphocytic leukemia
Annals of Hematology (2014)
-
Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus
Cell Communication and Signaling (2013)