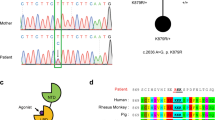
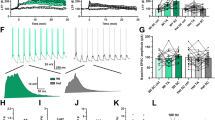
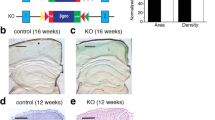
Abstract
Specific patterns of neuronal firing induce changes in synaptic strength that may contribute to learning and memory. If the postsynaptic NMDA (N-methyl-D-aspartate) receptors are blocked, long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission and the learning of spatial information are prevented. The NMDA receptor can bind a protein known as postsynaptic density-95 (PSD-95), which may regulate the localization of and/or signalling by the receptor. In mutant mice lacking PSD-95, the frequency function of NMDA-dependent LTP and LTD is shifted to produce strikingly enhanced LTP at different frequencies of synaptic stimulation. In keeping with neural-network models that incorporate bidirectional learning rules, this frequency shift is accompanied by severely impaired spatial learning. Synaptic NMDA-receptor currents, subunit expression, localization and synaptic morphology are all unaffected in the mutant mice. PSD-95 thus appears to be important in coupling the NMDA receptor to pathways that control bidirectional synaptic plasticity and learning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bear, M. F. & Malenka, R. C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 4, 389–399 (1994).
Bliss, T. V. P. & Collingridge, G. L. Asynaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Hollman, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994).
Nakanishi, S. & Masu, M. Molecular diversity and functions of glutamate receptors. Annu. Rev. Biophys. Biomolec. Struct. 23, 314–348 (1994).
Smart, T. G. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr. Opin. Neurobiol. 7, 358–367 (1997).
Kornau, H.-C., Schenker, L. T., Kennedy, M. B. & Seeburg, P. H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, 1737–1740 (1995).
Kim, E., Cho, K.-O., Rothschild, A. & Sheng, M. Heteromultimeriozation and NMDA receptor-clustering activity of chapsyn-110, a member of the PSD-95 family of proteins. Neuron 17, 103–113 (1996).
Müller, B. M. et al. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron 17, 255–265 (1996).
Niethammer, M., Kim, E. & Sheng, M. Interaction between the C terminus of NMDA receptor subunits and mutliple members of the PSD-95 family of membrane-associated guanylate kinases. J. Neurosci. 16, 2157–2163 (1996).
Cho, K. O., Hunt, C. A. & Kennedy, M. B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9, 929–942 (1992).
Kistner, U. et al. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J. Biol. Chem. 268, 4580–4583 (1993).
Kim, E., Niethammer, M., Rothschild, A., Jan, Y. N. & Sheng, M. Clustering of shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 378, 85–88 (1995).
Brenman, J. E. et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD–95 and alpha1-syntrophin mediated by PDZ domains. Cell 84, 757–767 (1996).
Hough, C. D., Woods, D. F., Park, S. & Bryant, P. J. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK discs-large. Genes Dev. 11, 3242–3252 (1997).
Müller, B. M. et al. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J. Neurosci. 15, 2354–2366 (1995).
Hunt, C. A., Schenker, L. J. & Kennedy, M. B. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane in forebrain synapses. J. Neurosci. 16, 1380–1388 (1996).
Wang, J. H. & Kelly, P. A. T. Attenuation of paired-pulse facilitation associated with synaptic potentiation mediated by postsynaptic mechanisms. J. Neurophysiol. 78, 2707–2716 (1997).
Irie, M. et al. Binding of neuroligins to PSD-95. Science 277, 1511–1515 (1997).
Tejedor, F. J. et al. Essential role for dlg in synaptic clustering of shaker K+ channels in vivo. J. Neurosci. 17, 152–159 (1997).
Mayford, M., Wang, J., Kandel, E. R. & O'Dell, T. J. CaMKII regulates the frequency-response function of hipocampal synapses for the production of both LTD and LTP. Cell 81, 891–904 (1995).
Dudek, S. M. & Bear, M. F. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J. Neurosci. 13, 2910–2918 (1993).
Morris, R. G. M., Anderson, E., Lynch, G. S. & Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776 (1986).
Tsien, J. Z., Huerta, P. T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996).
Allison, D. W., Gelfand, V. I., Spector, I. & Craig, A. M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: Differential attachment of NMDA versus AMPA receptors. J. Neurosci. 18, 2423–2436 (1998).
Wyszynski, M. et al. Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature 385, 439–442 (1997).
Wechsler, A. & Teichberg, V. I. Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J. 17, 3931–3939 (1998).
Bienenstock, E., Cooper, L. & Munro, P. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48 (1982).
Kim, J. H., Liao, D., Lau, L.-F. & Huganir, R. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 20, 683–691 (1998).
Chen, H.-J., Rojas-Soto, M., Oguni, A. & Kennedy, M. B. Asynaptic Ras GTPase-activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20, 895–904 (1998).
Naisbitt, S. et al. Characterization of guanylate kinase-associated protein, a postsynaptic density protein at excitatory synapses that interacts directly with postsynaptic density-95/synapse-associated protein 90. J. Neurosci. 17, 5687–5696 (1997).
Takeuchi, M. et al. SAPAPs: A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J. Biol. Chem. 272, 11943–11951 (1997).
Tsunoda, S. et al. Amultivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 388, 243–249 (1997).
Sejnowski, T. J. Statistical constraints on synaptic plasticity. J. Theor. Biol. 69, 387–389 (1977).
Willshaw, D. & Dayan, P. Optimal plasticity from matrix memories: what goes up must come down. Neural Comp. 2, 85–93 (1990).
Hancock, P. J. B., Smith, L. S. & Phillips, W. A. Abiologically supported error-correcting learning rule. Neural Comp. 3, 201–212 (1991).
Otto, T., Eichenbaum, H., Wiener, S. I. & Wible, C. G. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus 1, 181–192 (1991).
Morrison, B. M., Janssen, W. G. M., Gordon, J. W. & Morrison, J. H. Light and electron microscopic distribution of the AMPA receptor subunit, GluR2, in the spinal cord of control and G86R mutant superoxide dismutase transgenic mice. J. Comp. Neurol. 395, 523–534 (1998).
Rayport, S. et al. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J. Neurosci. 12, 4264–4280 (1992).
Acknowledgements
We thank A. J. H. Smith for advice and for the 129 genomic library, J. Ure for embryo injection, L. Anderson and J. Young for mouse care, W. Janssen for technical assistance, F. Johnston and G.Brown for photography, and R. Ellaway for illustrations. This work was supported by the Fyssen Foundation (M.M.) and the BBSRC (M.M., M.D. and P.C.), by grants from the NIH and the Charles A. Dana Foundation (J.H.M. and Y.H.), the Esther A. and Joseph Klingenstein Fund and the Pew Charitable Trusts (T.J.O.), a grant from the National Institute of Mental Health (T.J.O.), and the Wellcome Trust (L.C.W. and S.G.N.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migaud, M., Charlesworth, P., Dempster, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 (1998). https://doi.org/10.1038/24790
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/24790
This article is cited by
-
Adnp-mutant mice with cognitive inflexibility, CaMKIIα hyperactivity, and synaptic plasticity deficits
Molecular Psychiatry (2023)
-
Latent toxoplasmosis impairs learning and memory yet strengthens short-term and long-term hippocampal synaptic plasticity at perforant pathway-dentate gyrus, and Schaffer collatterals-CA1 synapses
Scientific Reports (2023)
-
Alcohol Consumption During Adolescence Alters the Cognitive Function in Adult Male Mice by Persistently Increasing Levels of DUSP6
Molecular Neurobiology (2023)
-
Extracellular Vesicle-Serpine-1 Affects Neural Progenitor Cell Mitochondrial Networks and Synaptic Density: Modulation by Amyloid Beta and HIV-1
Molecular Neurobiology (2023)
-
LOTUS suppresses amyloid β-induced dendritic spine elimination through the blockade of amyloid β binding to PirB
Molecular Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.