Abstract
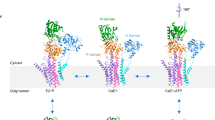
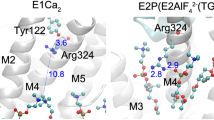
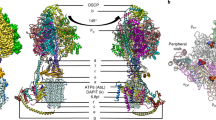
Calcium ATPase is a member of the P-type ATPases that transport ions across the membrane against a concentration gradient. Here we have solved the crystal structure of the calcium ATPase of skeletal muscle sarcoplasmic reticulum (SERCA1a) at 2.6 Å resolution with two calcium ions bound in the transmembrane domain, which comprises ten α-helices. The two calcium ions are located side by side and are surrounded by four transmembrane helices, two of which are unwound for efficient coordination geometry. The cytoplasmic region consists of three well separated domains, with the phosphorylation site in the central catalytic domain and the adenosine-binding site on another domain. The phosphorylation domain has the same fold as haloacid dehalogenase. Comparison with a low-resolution electron density map of the enzyme in the absence of calcium and with biochemical data suggests that large domain movements take place during active transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ebashi, S. & Lipman, F. Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J. Cell. Biol. 14, 389–400 (1962).
Møller, J. V., Juul, B. & le Maire, M. Structural organization, ion transport, and energy transduction of P- type ATPases. Biochim. Biophys. Acta 1286, 1–51 (1996).
MacLennan, D. H., Rice, W. J. & Green, N. M. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 272, 28815–28818 ( 1997).
McIntosh, D. B. The ATP binding sites of P-type ion transport ATPases. Adv. Mol. Cell. Biol. 23A, 33–99 ( 1998).
MacLennan, D. H., Brandl, C. J., Korczak, B. & Green, N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 316, 696– 700 (1985).
Clarke, D. M., Loo, T. W., Inesi, G. & MacLennan, D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+- ATPase. Nature 339, 476–478 ( 1989).
Saraste, M., Sibbald, P. R. & Wittinghofer, A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15, 430– 434 (1990).
Aravind, L., Galperin, M. Y. & Koonin, E. V. The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem. Sci. 23, 127–129 (1998).
Dux, L. & Martonosi, A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J. Biol. Chem. 258, 2599–2603 (1983).
Dux, L., Pikula, S., Mullner, N. & Martonosi, A. Crystallization of Ca2+-ATPase in detergent-solubilized sarcoplasmic reticulum. J. Biol. Chem. 262, 6439– 6442 (1987).
Stokes, D. L. & Green, N. M. Three-dimensional crystals of CaATPase from sarcoplasmic reticulum. Symmetry and molecular packing. Biophys. J. 57, 1–14 ( 1990).
Toyoshima, C., Sasabe, H. & Stokes, D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature 362, 467–471 ( 1993).
Zhang, P., Toyoshima, C., Yonekura, K., Green, N. M. & Stokes, D. L. Structure of the calcium pump from sarcoplasmic reticulum at 8-Å resolution. Nature 392, 835–839 (1998).
Ogawa, H., Stokes, D. L., Sasabe, H. & Toyoshima, C. Structure of the Ca2+ pump of sarcoplasmic reticulum: a view along the lipid bilayer at 9-Å resolution. Biophys. J. 75, 41–52 (1998).
Brandl, C. J., deLeon, S., Martin, D. R. & MacLennan, D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J. Biol. Chem. 262, 3768–3774 (1987).
Toyofuku, T., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Amino acids Lys-Asp-Asp-Lys-Pro-Val402 in the Ca2+-ATPase of cardiac sarcoplasmic reticulum are critical for functional association with phospholamban. J. Biol. Chem. 269, 22929–22932 (1994).
Yu, M. et al. Specific substitutions at amino acid 256 of the sarcoplasmic/endoplasmic reticulum Ca2+ transport ATPase mediate resistance to thapsigargin in thapsigargin-resistant hamster cells. J. Biol. Chem. 273, 3542–3546 (1998).
Nayal, M. & Di Cera, E. Predicting Ca2+-binding sites in proteins. Proc. Natl Acad. Sci. USA 91, 817–821 (1994).
Inesi, G., Kurzmack, M., Coan, C. & Lewis, D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J. Biol. Chem. 255, 3025–3031 (1980).
Soulié, S. et al. NMR conformational study of the sixth transmembrane segment of sarcoplasmic reticulum Ca2+-ATPase. Biochemistry 38, 5813–5821 ( 1999).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Clarke, D. M. et al. Functional consequences of glutamate, aspartate, glutamine, and asparagine mutations in the stalk sector of the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 264, 11246–11251 (1989).
Hisano, T. et al. Crystal structure of L-2-haloacid dehalogenase from Pseudomonas sp. YL. An α/β hydrolase structure that is different from the α/β hydrolase fold. J. Biol. Chem. 271, 20322–20330 (1996).
McIntosh, D. B., Woolley, D. G., Vilsen, B. & Andersen, J. P. Mutagenesis of segment 487Phe-Ser-Arg-Asp-Arg-Lys492 of sarcoplasmic reticulum Ca2+-ATPase produces pumps defective in ATP binding. J. Biol. Chem. 271, 25778–25789 (1996).
Pick, U. Interaction of fluorescein isothiocyanate with nucleotide-binding sites of the Ca-ATPase from sarcoplasmic reticulum. Eur. J. Biochem. 121, 187–195 (1981).
McIntosh, D. B., Woolley, D. G. & Berman, M. C. 2′,3′-O-(2,4,6-trinitrophenyl)-8-azido-AMP and-ATP photolabel Lys-492 at the active site of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 267, 5301 –5309 (1992).
Yamamoto, H., Imamura, Y., Tagaya, M., Fukui, T. & Kawakita, M. Ca2+-dependent conformational change of the ATP-binding site of Ca2+-transporting ATPase of sarcoplasmic reticulum as revealed by an alteration of the target-site specificity of adenosine triphosphopyridoxal. J. Biochem. (Tokyo) 106, 1121–1125 (1989).
Juul, B. et al. Do transmembrane segments in proteolyzed sarcoplasmic reticulum Ca2+- ATPase retain their functional Ca2+ binding properties after removal of cytoplasmic fragments by proteinase K? J. Biol. Chem. 270, 20123–20134 (1995).
Andersen, J. P., Vilsen, B., Collins, J. H. & Jørgensen, P. L. Localization of E1–E2 conformational transitions of sarcoplasmic reticulum Ca-ATPase by tryptic cleavage and hydrophobic labeling. J. Membr. Biol. 93, 85–92 ( 1986).
Li, Y. F. et al. Crystal structures of reaction intermediates of L-2-haloacid dehalogenase and implications for the reaction mechanism. J. Biol. Chem. 273, 15035–15044 ( 1998).
Goldshleger, R. & Karlish, S. J. D. Fe-catalyzed cleavage of the alpha subunit of Na/K-ATPase: evidence for conformation-sensitive interactions between cytoplasmic domains. Proc. Natl Acad. Sci. USA 94, 9596-9601 (1997).
Rice, W. J., Green, N. M. & MacLennan, D. H. Site-directed disulfide mapping of helices M4 and M6 in the Ca2+ binding domain of SERCA1a, the Ca2+ ATPase of fast twitch skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 272, 31412–31419 (1997).
Falson, P. et al. The cytoplasmic loop between putative transmembrane segments 6 and 7 in sarcoplasmic reticulum Ca2+-ATPase binds Ca2+ and is functionally important. J. Biol. Chem. 272, 17258–17262 (1997).
McIntosh, D. B. Glutaraldehyde cross-links Lys-492 and Arg-678 at the active site of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 267, 22328–22335 (1992).
Nakamoto, R. K. & Inesi, G. Studies of the interactions of 2′,3′-O-(2,4,6- trinitrocyclohexyldienylidine)adenosine nucleotides with the sarcoplasmic reticulum (Ca2+ + Mg2+)-ATPase active site. J. Biol. Chem. 259, 2961–2970 (1984).
Huang, S. & Squier, T. C. Enhanced rotational dynamics of the phosphorylation domain of the Ca- ATPase upon calcium activation. Biochemistry 37, 18064–18073 (1998).
Champeil, P. et al. Characterization of a protease-resistant domain of the cytosolic portion of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 273, 6619–6631 (1998).
Sagara, Y. & Inesi, G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 266, 13503– 13506 (1991).
Misra, M., Taylor, D., Oliver, T. & Taylor, K. Effect of organic anions on the crystallization of the Ca2+-ATPase of muscle sarcoplasmic reticulum. Biochim. Biophys. Acta 1077, 107–118 (1991).
Kamiya, N.et al. Design of the high energy undulator pilot beamline for macromolecular crystallography at the SPring-8. Rev. Sci. Instrum. 66, 1703–1705 (1995).
Adachi, S., Oguchi, T. & Ueki, T. Present status of RIKEN beamline II (BL44B2) for structural biology. SPring-8 Annual Report 1, 239– 240 (1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–325 ( 1997).
Leslie, A. G. W. MOSFLM- recent changes and future developments. CCP4 Newsletter on Protein Crystallography 35, 18– 19 (1998).
Collaborative Computational project, No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 ( 1994).
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946 –950 (1991).
Clarke, D. M., Loo, T. W. & MacLennan, D. H. Functional consequences of alterations to amino acids located in the nucleotide binding domain of the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 265, 22223–22227 (1990).
Ovchinnikov, Y. et al. Affinity modification of E1-form of Na+, K+-ATPase revealed Asp-710 in the catalytic site. FEBS Lett. 217, 111–116 ( 1987).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Acknowledgements
We thank S. Adachi, N. Kamiya (Riken) and M. Kawamoto (JASRI) for their help in data taking at SPring-8, and K. Tani for computations. We acknowledge that very first crystals for X-ray were made by H. Mukai. This work was supported in part by grants-in-aid from the Ministry of Culture, Education, Science and Sports of Japan and from CRESTO (to C.T. and M.N.) and also by Toray Science Foundation (C.T.). This paper is dedicated to S. Ebashi, a founder of Ca2+-ATPase field and the father of the calcium theory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toyoshima, C., Nakasako, M., Nomura, H. et al. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000). https://doi.org/10.1038/35015017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35015017
This article is cited by
-
Analysis of cholesterol-recognition motifs of the plasma membrane Ca2+-ATPase
Journal of Bioenergetics and Biomembranes (2024)
-
Effect of nitrosyl iron complexes and their thio ligands on the activity of phosphodiesterase and sarcoplasmic reticulum Ca2+-ATPase
Russian Chemical Bulletin (2023)
-
Thyroid Hormones and Skeletal Muscle Beyond Thermogenesis
Journal of Science in Sport and Exercise (2023)
-
Bioinformatics approaches for classification and investigation of the evolution of the Na/K-ATPase alpha-subunit
BMC Ecology and Evolution (2022)
-
Electrostatic interactions between single arginine and phospholipids modulate physiological properties of sarcoplasmic reticulum Ca2+-ATPase
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.