Abstract
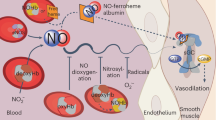
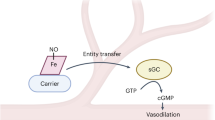
Previous studies support a model in which the physiological O2 gradient is transduced by haemoglobin into the coordinate release from red blood cells of O2 and nitric oxide (NO)-derived vasoactivity to optimize oxygen delivery in the arterial periphery1,2. But whereas both O2 and NO diffuse into red blood cells, only O2 can diffuse out3,4,5. Thus, for the dilation of blood vessels by red blood cells, there must be a mechanism to export NO-related vasoactivity, and current models of NO-mediated intercellular communication should be revised. Here we show that in human erythrocytes haemoglobin-derived S-nitrosothiol (SNO), generated from imported NO, is associated predominantly with the red blood cell membrane, and principally with cysteine residues in the haemoglobin-binding cytoplasmic domain of the anion exchanger AE1. Interaction with AE1 promotes the deoxygenated structure in SNO–haemoglobin, which subserves NO group transfer to the membrane. Furthermore, we show that vasodilatory activity is released from this membrane precinct by deoxygenation. Thus, the oxygen-regulated cellular mechanism that couples the synthesis and export of haemoglobin-derived NO bioactivity operates, at least in part, through formation of AE1–SNO at the membrane–cytosol interface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380, 221–226 (1996).
Stamler, J. S. et al. Blood flow regulation by S-nitrosohemoglobin is controlled by the physiological oxygen gradient. Science 276, 2034–2037 (1997).
Gow, A. J. & Stamler, J. S. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391, 169–173 (1998).
Gow, A. J., Luchsinger, B. P., Pawloski, J. R., Singel, D. J. & Stamler, J. S. The oxyhemoglobin reaction of nitric oxide. Proc. Natl Acad. Sci. USA 96, 9027–9032 (1999).
McMahon, T. J., Stone, A. E., Bonaventura, J., Singel, D. J. & Stamler, J. S. Functional coupling of oxygen-binding and vasoactivity in S-nitrosohemoglobin. J. Biol. Chem. 275, 16738–16745 (2000).
McMahon, T. J. & Stamler, J. S. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 301, 99–114 (1999).
Pawloski, J. R., Swaminathan, R. V. & Stamler, J. S. Cell-free and erythrocytic S-nitrosohemoglobin inhibits human platelet aggregation. Circulation 97, 263–267 (1998).
Rauenbuehler, P. B., Cordes, K. A. & Salhany, J. M. Identification of the hemoglobin binding sites on the inner surface of the erythrocyte membrane. Biochim. Biophys. Acta 692, 361–370 (1982).
Walder, J. A. et al. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J. Biol. Chem. 259, 10238–10246 (1984).
Low, P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane–peripheral protein interactions. Biochim. Biophys. Acta 864, 145–167 (1986).
Kondo, T. Preparation of microcapsules from human erythrocytes: use in transport experiments of glutathione and its S-conjugate. Methods Enzymol. 171, 217–225 (1989).
Stamler, J. S., Toone, E. J., Lipton, S. A. & Sucher, N. J. (S)NO signals: translocation, regulation, and a consensus motif. Neuron 18, 691–696 (1997).
Sayare, M. & Fikiet, M. Cross-linking of hemoglobin to the cytoplasmic surface of human erythrocyte membranes. Identification of band 3 as a site for hemoglobin binding in Cu2+-o-phenanthroline catalyzed cross-linking. J. Biol. Chem. 256, 13152–13158 (1981).
Liu, S. Q. & Knauf, P. A. Lys-430, site of irreversible inhibition of band 3 Cl- flux by eosin-5-maleimide, is not at the transport site. Am. J. Physiol. 264, C1155–C1164 (1993).
Falke, J. J. & Chan, S. I. Molecular mechanisms of band 3 inhibitors. 1. Transport site inhibitors. Biochemistry 25, 7888–7894 (1986).
Okubo, K., Kang, D., Hamasaki, N. & Jennings, M. L. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J. Biol. Chem. 269, 1918–1926 (1994).
Salhany, J. M., Cordes, K. A. & Gaines, E. D. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry 19, 1447–1454 (1980).
Hsu, L. & Morrison, M. The interaction of human erythrocyte band 3 with cytoskeletal proteins. Archiv. Biochem. Biophys. 227, 31–38 (1983).
Macara, I. G., Kuo, S. & Cantley, L. C. Evidence that inhibitors of anion exchange induce a transmembrane conformational change in band 3. J. Biol. Chem. 258, 1785–1792 (1983).
Falke, J. J. & Chan, S. I. Molecular mechanisms of band 3 inhibitors. 3. Translocation inhibitors. Biochemistry 25, 7899–7906 (1986).
Chetrite, G. & Cassoly, R. Affinity of hemoglobin for the cytoplasmic fragment of human erythrocyte membrane band 3. Eqilibrium measurements at physiological pH using matrix-bound proteins: the effects of ionic strength, deoxygenation and of 2,3,-diphosphoglycerate. J. Mol. Biol. 185, 639–644 (1985).
Galanter, W. L. & Labotka, R. J. The binding of nitrate to the human anion exchange protein (AE1) studied with 14C nuclear magnetic resonance. Biochim. Biophys. Acta 1079, 146–151 (1991).
Shingles, R., Roh, M. H. & McCarty, R. E. Direct measurement of nitrite transport across erythrocyte membrane vesicles using the fluorescent probe, 6-methoxy-N-(3-sulfopropyl) quinolinium. J. Bioenerg. Biomembr. 29, 611–616 (1997).
Soszynski, M. & Bartosz, G. Penetration of erythrocyte membrane by peroxynitrite: participation of the anion exchange protein. Biochem. Mol. Biol. Int. 43, 319–325 (1997).
Czerwinski, M. et al. Degradation of the human erythrocyte membrane band 3 studied with the monoclonal antibody directed against an epitope on the cytoplasmic fragment of band 3. Eur. J. Biochem. 174, 647–654 (1988).
Telen, M. J., Scearce, R. M. & Haynes, B. Human erythrocyte antigens. III. Characterization of a panel of murine monoclonal antibodies that react with human erythrocyte and erythroid precursor membranes. Vox Sang. 52, 236–243 (1987).
Liu, X. et al. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 273, 18709–18713 (1998).
Vaughn, M. W., Huang, K.-T., Kuo, L. & Liao, J. C. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 275, 2342–2348 (2000).
Acknowledgements
We are grateful to M. Telen for providing antibody to AE1 and for advice. This work was supported by an AHA postdoctoral fellowship to J.R.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pawloski, J., Hess, D. & Stamler, J. Export by red blood cells of nitric oxide bioactivity. Nature 409, 622–626 (2001). https://doi.org/10.1038/35054560
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35054560
This article is cited by
-
Combining nitric oxide and calcium sensing for the detection of endothelial dysfunction
Communications Chemistry (2023)
-
Erythrocytes from patients with ST-elevation myocardial infarction induce cardioprotection through the purinergic P2Y13 receptor and nitric oxide signaling
Basic Research in Cardiology (2022)
-
Endothelial alpha globin is a nitrite reductase
Nature Communications (2022)
-
Assessment of transient changes in oxygen diffusion of single red blood cells using a microfluidic analytical platform
Communications Biology (2021)
-
Nitrite in breast milk: roles in neonatal pathophysiology
Pediatric Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.