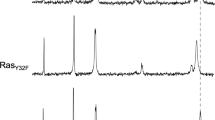
Abstract
AN early response to the tyrosine kinase activity of v-Src is an increase in phospholipase D (PLD) activity1, which leads to the generation of biologically active lipid second messengers, including phosphatidic acid, lysophosphatidic acid and diacylglycerol2. We have recently demonstrated that v-Src-induced PLD activity is mediated by Ras3, although Ras involvement was indirect, requiring a cytosolic factor for PLD activation3. Ras interacts with4–6 and activates Ral–GDS13, the exchange factor responsible for the activation of Ral GTPases. Here we report that this newly identified Ras/Ral signalling pathway mediates PLD activation by v-Src. PLD activity could be precipitated from v-Src-transformed cell lysates with immobilized RalA protein and with an anti-Ral antibody. A mutation to the region of RalA analogous to the 'effector domain' of Ras did not reduce the ability of RalA to complex with PLD, although deletion of a Ral-specific amino-terminal region did. Overexpression of RalA potentiated PLD activation by v-Src, and expression of dominant negative RalA mutants inhibited both v-Src- and v-Ras-induced PLD activity. Thus RalA is involved in the tyrosine kinase activation of PLD through its unique N terminus, and that PLD is a downstream target of a Ras/Ral GTPase cascade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Song, J., Pfeffer, L. M. & Foster, D. A. Molec. cell. Biol. 11, 4903–4908 (1991).
Foster, D. A. Cell Signalling 5, 389–399 (1993).
Jiang, H., Lu, Z., Luo, J. Q., Wolfman, A. & Foster, D. A. J. biol. Chem. 270, 6006–6009 (1995).
Kikuchi, A., Demo, S. D., Ye, Z., Chen, Y. & Williams, L. T. Molec. cell. Biol. 14, 7483–7491 (1994).
Hofer, F., Fields, S., Schneider, C. & Martin, G. S. Proc. natn. Acad. Sci. U.S.A. 91, 11089–11093 (1994).
Spaargaren, M. & Bischoff, J. R. Proc. natn. Acad. Sci. U.S.A. 91, 12609–12613 (1994).
Moodie, S. A., Willumsen, B. M., Weber, M. J. & Wolfman, A. Science 260, 1658–1661 (1993).
Vojtek, A. B., Holenberg, S. M. & Cooper, J. A. Cell 74, 205–214 (1993).
Rodriguez-Viciana, P. et al. Nature 370, 527–532 (1994).
Cantor, S., Urano, T. & Feig, L. A. Molec. cell. Biol. 15, 4578–4584 (1995).
Feig, L. A. & Cooper, G. M. Molec. cell. Biol. 8, 3235–3243 (1988).
Farnsworth, C. L. & Feig, L. A. Molec. cell. Biol. 11, 4822–4829 (1991).
Urano, T., Emkey, R. & Feig, L. A. EMBO J. (in the press).
Song, J. & Foster, D. A. Biochem. J. 294, 711–717 (1993).
Carnero, A., Cuadrado, A., del Peso, L. & Lacal, J. C. Oncogene 9, 1387–1395 (1994).
Bowman, E. P., Uhlinger, D. J. & Lambeth, J. D. J. biol. Chem. 268, 21509–21512 (1993).
Malcolm, K. C., Ross, A. H., Qui, R.-G., Symons, M. & Exton, J. E. J. biol. Chem. 269, 25951–25954 (1994).
Siddiqi, A. R. et al. J. biol. Chem. 270, 8466–8473 (1995).
Brown, H. A., Gutowski, S., Moomaw, C. R., Slaughter, C. & Stirnweis, P. C. Cell 75, 1137–1144 (1993).
Cockroft, S. et al. Science 263, 523–526 (1994).
Lambeth, J. D. et al. J. biol. Chem. 270, 2431–2434 (1995).
Ridley, A. J. & Hall, A. Cell 70, 389–399 (1992).
Furth, M. E., Davis, L. J., Fleurdelys, B. & Scolnick, E. M. J. Virol. 43, 294–304 (1982).
Emkey, R., Friedman, S. & Feig, L. A. J. biol. Chem. 266, 9703–9706 (1991).
Qureshi, S. A. et al. J. biol. Chem. 267, 17635–17639 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jiang, H., Luo, JQ., Urano, T. et al. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature 378, 409–412 (1995). https://doi.org/10.1038/378409a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/378409a0
This article is cited by
-
FOXD1-dependent RalA-ANXA2-Src complex promotes CTC formation in breast cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
K15 promoter-driven enforced expression of NKIRAS exhibits tumor suppressive activity against the development of DMBA/TPA-induced skin tumors
Scientific Reports (2021)
-
NMR resonance assignments for the active and inactive conformations of the small G protein RalA
Biomolecular NMR Assignments (2020)
-
The RAS-RAL axis in cancer: evidence for mutation-specific selectivity in non-small cell lung cancer
Acta Pharmacologica Sinica (2015)
-
Phospholipase D (PLD) drives cell invasion, tumor growth and metastasis in a human breast cancer xenograph model
Oncogene (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.