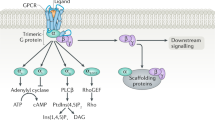
Abstract
Understanding precisely how the heart can recognize and respond to many different extracellular signalling molecules, such as neurotransmitters, hormones and growth factors, will aid the identification of new therapeutic targets through which cardiovascular diseases can be combated. In recent years, we have learned more about the complex interactions that occur between the receptors and the signalling pathways of the heart and its environment. Most of these discoveries have focused on the most common type of cardiac receptor — the seven-transmembrane-spanning receptor or G-protein-coupled receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoffman, B. B. & Lefkowitz, R. J. in Goodman and Gilman's The Pharmacological Basis of Therapeutics 9th edn (eds Hardman, J. G., Gilman, A. G. & Limbird, L. E.) 199–248 (McGraw-Hill, New York, 1996).
Clapham, D. E. & Neer, E. J. G protein βγ subunits. Annu. Rev. Pharmacol. Toxicol. 37, 167–203 (1997).
Molkentin, J. D. & Dorn, I. G. II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 63, 391–426 (2001).
Lefkowitz, R. J. G protein-coupled receptors. III. New roles for receptor kinases and β-ARrestins in receptor signaling and desensitization. J. Biol. Chem. 273, 18677–18680 (1998).
Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 67, 653–692 (1998).
Laporte, S. A., Oakley, R. H., Holt, J. A., Barak, L. S. & Caron, M. G. The interaction of β-ARrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem. 275, 23120–23126 (2000).
DeFea, K. A. et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-ARrestin-dependent scaffolding complex. Proc. Natl Acad. Sci. USA 97, 11086–11091 (2000).
Luttrell, L. M. et al. β-ARrestin-dependent formation of β2 adrenergic receptor–Src protein kinase complexes. Science 283, 655–661 (1999).
DeFea, K. A. et al. β-ARrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148, 1267–1281 (2000).
Schmid, S. L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66, 511–548 (1997).
Luttrell, L. M. et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl Acad. Sci. USA 98, 2449–2454 (2001).
McDonald, P. H. et al. β-ARrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 (2000).
Shenoy, S. K., McDonald, P. H., Kohout, T. A. & Lefkowitz, R. J. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-ARrestin. Science 294, 1307–1313 (2001).
Chien, K. R. Stress pathways and heart failure. Cell 98, 555–558 (1999).
Esposito, G. et al. Cardiac overexpression of a Gq inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activity in in vivo pressure overload. Circulation 103, 1453–1458 (2001).
Rapacciuolo, A. et al. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy. J. Am. Coll. Cardiol. 38, 876–882 (2001).
Naga Prasad, S. V., Esposito, G., Mao, L., Koch, W. J. & Rockman, H. A. Gβγ-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J. Biol. Chem. 275, 4693–4698 (2000).
Choi, D. J., Koch, W. J., Hunter, J. J. & Rockman, H. A. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J. Biol. Chem. 272, 17223–17229 (1997).
Knowlton, K. U. et al. The α1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J. Biol. Chem. 268, 15374–15380 (1993).
Sugden, P. H. Signaling in myocardial hypertrophy: life after calcineurin? Circ. Res. 84, 633–646 (1999).
D'Angelo, D. D. et al. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc. Natl Acad. Sci. USA 94, 8121–8126 (1997).
Hein, L. et al. Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block. Proc. Natl Acad. Sci. USA 94, 6391–6396 (1997).
Choukroun, G. et al. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH2-terminal kinases. J. Clin. Invest. 104, 391–398 (1999).
Rockman, H. A. et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl Acad. Sci. USA 88, 8277–8281 (1991).
Akhter, S. A. et al. Targeting the receptor–Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280, 574–577 (1998).
Grossman, W., Jones, D. & McLaurin, L. P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 56, 56–64 (1975).
Levy, D., Garrison, R. J., Savage, D. D., Kannel, W. B. & Castelli, W. P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Engl. J. Med. 322, 1561–1566 (1990).
Esposito, G. et al. Genetic alterations that inhibit in vivo pressure overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 105, 85–92 (2002).
Leimbach, W. N. Jr et al. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73, 913–919 (1986).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Bristow, M. R. Why does the myocardium fail? Insights from basic science. Lancet 352 (Suppl. I), 8–14 (1998).
Packer, M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 20, 248–254 (1992).
Ungerer, M. et al. Expression of β-ARrestins and β-adrenergic receptor kinases in the failing human heart. Circ. Res. 74, 206–213 (1994).
Feldman, A. M. et al. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J. Clin. Invest. 82, 189–197 (1988).
Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997).
Zhu, W. Z. et al. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl Acad. Sci. USA 98, 1607–1612 (2001).
Green, S. A., Cole, G., Jacinto, M., Innis, M. & Liggett, S. B. A polymorphism of the human β2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J. Biol. Chem. 268, 23116–23121 (1993).
Podlowski, S. et al. β1-adrenoceptor gene variations: a role in idiopathic dilated cardiomyopathy? J. Mol. Med. 78, 87–93 (2000).
Liggett, S. B. et al. The Ile164 β2-adrenergic receptor polymorphism adversely affects the outcome of congestive heart failure. J. Clin. Invest. 102, 1534–1539 (1998).
Wagoner, L. E. et al. Polymorphisms of the β2-adrenergic receptor determine exercise capacity in patients with heart failure. Circ. Res. 86, 834–840 (2000).
Collins, F. S. Shattuck lecture—medical and societal consequences of the human genome project. N. Engl. J. Med. 341, 28–37 (1999).
Epstein, S. E. & Braunwald, E. The effect of β-adrenergic blockade on patterns of urinary sodium excretion. Studies in normal subjects and in patients with heart disease. Ann. Intern. Med. 65, 20–27 (1966).
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353, 9–13 (1999).
Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 353, 2001–2007 (1999).
Packer, M. et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 334, 1349–1355 (1996).
Packer, M. et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658 (2001).
Australia/New Zealand Heart Failure Research Collaborative Group. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 349, 375–380 (1997).
Esposito, G. et al. Cellular and functional defects in a mouse model of heart failure. Am. J. Physiol. Heart Circ. Physiol. 279, H3101–H3112 (2000).
Koch, W. J., Lefkowitz, R. J. & Rockman, H. A. Functional consequences of altering myocardial adrenergic receptor signaling. Annu. Rev. Physiol. 62, 237–260 (2000).
Engelhardt, S., Hein, L., Wiesmann, F. & Lohse, M. J. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl Acad. Sci. USA 96, 7059–7064 (1999).
Milano, C. A. et al. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science 264, 582–586 (1994).
Liggett, S. B. et al. Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 101, 1707–1714 (2000).
Rohrer, D. K. Physiological consequences of β-adrenergic receptor disruption. J. Mol. Med. 76, 764–772 (1998).
Chruscinski, A. J. et al. Targeted disruption of the β2 adrenergic receptor gene. J. Biol. Chem. 274, 16694–16700 (1999).
Communal, C., Singh, K., Sawyer, D. B. & Colucci, W. S. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100, 2210–2212 (1999).
Dorn, G. W. II Tepe, N. M., Lorenz, J. N., Koch, W. J. & Liggett, S. B. Low- and high-level transgenic expression of β2-adrenergic receptors differentially affect cardiac hypertrophy and function in Gαq-overexpressing mice. Proc. Natl Acad. Sci. USA 96, 6400–6405 (1999).
Rockman, H. A. et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA 95, 7000–7005 (1998).
Freeman, K. et al. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J. Clin. Invest. 107, 967–974 (2001).
Akhter, S. A. et al. Restoration of β-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proc. Natl Acad. Sci. USA 94, 12100–12105 (1997).
Maurice, J. P. et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary β2-adrenergic receptor gene delivery. J. Clin. Invest. 104, 21–29 (1999).
Shah, A. S. et al. Intracoronary adenovirus-mediated delivery and overexpression of the β2-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation 101, 408–414 (2000).
Minamisawa, S. et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 99, 313–322 (1999).
Roth, D. M. et al. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation 99, 3099–3102 (1999).
Koch, W. J. et al. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a β-ARK inhibitor. Science 268, 1350–1353 (1995).
Jaber, M. et al. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl Acad. Sci. USA 93, 12974–12979 (1996).
Rockman, H. A. et al. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc. Natl Acad. Sci. USA 93, 9954–9959 (1996).
Rockman, H. A. et al. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 273, 18180–18184 (1998).
Arber, S. et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88, 393–403 (1997).
Cho, M. C. et al. Defective β-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. J. Biol. Chem. 274, 22251–22256 (1999).
Freeman, K. et al. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. Am. J. Physiol. Heart Circ. Physiol. 280, H151–H159 (2001).
Harding, V. B., Jones, L. R., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. Cardiac β ARK1 inhibition prolongs survival and augments β-blocker therapy in a mouse model of severe heart failure. Proc. Natl Acad. Sci. USA 98, 5809–5814 (2001).
White, D. C. et al. Preservation of myocardial β-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc. Natl Acad. Sci. USA 97, 5428–5433 (2000).
Shah, A. S. et al. In vivo ventricular gene delivery of a β-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation 103, 1311–1316 (2001).
Naga Prasad, S. V., Barak, L. S., Rapacciuolo, A., Caron, M. G. & Rockman, H. A. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by β-adrenergic receptor Kinase 1. A role in receptor sequestration. J. Biol. Chem. 276, 18953–18959 (2001).
The Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. Lancet 336, 1–6 (1990).
Iaccarino, G., Tomhave, E. D., Lefkowitz, R. J. & Koch, W. J. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation 98, 1783–1789 (1998).
McNamara, D. M. et al. Pharmacogenetic interactions between β-blocker therapy and the angiotensin-converting enzyme deletion polymorphism in patients with congestive heart failure. Circulation 103, 1644–1648 (2001).7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rockman, H., Koch, W. & Lefkowitz, R. Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002). https://doi.org/10.1038/415206a
Issue Date:
DOI: https://doi.org/10.1038/415206a
This article is cited by
-
Circulating acetylcholine serves as a potential biomarker role in pulmonary hypertension
BMC Pulmonary Medicine (2024)
-
Autonomic and circulatory alterations persist despite adequate resuscitation in a 5-day sepsis swine experiment
Scientific Reports (2022)
-
MiR-150 blunts cardiac dysfunction in mice with cardiomyocyte loss of β1-adrenergic receptor/β-arrestin signaling and controls a unique transcriptome
Cell Death Discovery (2022)
-
Cell therapy rescues aging-induced beta-1 adrenergic receptor and GRK2 dysfunction in the coronary microcirculation
GeroScience (2022)
-
The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review
Annals of Intensive Care (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.