Abstract
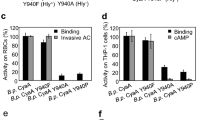
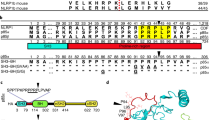
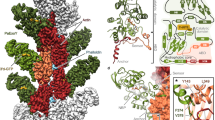
Oedema factor, a calmodulin-activated adenylyl cyclase, is important in the pathogenesis of anthrax. Here we report the X-ray structures of oedema factor with and without bound calmodulin. Oedema factor shares no significant structural homology with mammalian adenylyl cyclases or other proteins. In the active site, 3′-deoxy-ATP and a single metal ion are well positioned for catalysis with histidine 351 as the catalytic base. This mechanism differs from the mechanism of two-metal-ion catalysis proposed for mammalian adenylyl cyclases. Four discrete regions of oedema factor form a surface that recognizes an extended conformation of calmodulin, which is very different from the collapsed conformation observed in other structures of calmodulin bound to effector peptides. On calmodulin binding, an oedema factor helical domain of relative molecular mass 15,000 undergoes a 15 Å translation and a 30° rotation away from the oedema factor catalytic core, which stabilizes a disordered loop and leads to enzyme activation. These allosteric changes provide the first molecular details of how calmodulin modulates one of its targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Leppla, S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl Acad. Sci. USA 79, 3162–3166 (1982).
Ladant, D. & Ullmann, A. Bordatella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7, 172–176 (1999).
Yahr, T. L., Vallis, A. J., Hancock, M. K., Barbieri, J. T. & Frank, D. W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl Acad. Sci. USA 95, 13899–13904 (1998).
Duesbery, N. S. et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 (1998).
Dixon, T. C., Meselson, M., Guillemin, J. & Hanna, P. C. Anthrax. N. Engl. J. Med. 341, 815–826 (1999).
Eldik, L. V. & Watterson, D. M. Calmodulin and Signal Transduction (Academic, New York, 1998).
Drum, C. L. et al. An extended conformation of calmodulin induces interactions between the structural domains of adenylyl cyclase from Bacillus anthracis to promote catalysis. J. Biol. Chem. 275, 36334–36340 (2000).
Tang, W. J., Krupinski, J. & Gilman, A. G. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J. Biol. Chem. 266, 8595–8603 (1991).
Deisseroth, K., Heist, E. K. & Tsien, R. W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392, 198–202 (1998).
Peters, C. & Mayer, A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575–580 (1998).
DeMaria, C. D., Soong, T. W., Alseikhan, B. A., Alvania, R. S. & Yue, D. T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411, 484–489 (2001).
Babu, Y. S. et al. Three-dimensional structure of calmodulin. Nature 315, 37–40 (1985).
Kuboniwa, H. et al. Solution structure of calcium-free calmodulin. Nature Struct. Biol. 2, 768–776 (1995).
Finn, B. E. et al. Calcium-induced structural changes and domain autonomy in calmodulin. Nature Struct. Biol. 2, 777–83 (1995).
Meador, W. E., Means, A. R. & Quiocho, F. A. Target enzyme recognition by calmodulin: 2.4 Å structure of a calmodulin–peptide complex. Science 257, 1251–1255 (1992).
Meador, W. E., Means, A. R. & Quiocho, F. A. Modulation of calmodulin plasticity in molecular recognition on the basis of X-ray structures. Science 262, 1718–1721 (1993).
Osawa, M. et al. A novel target recognition revealed by calmodulin in complex with Ca2+/calmodulin-dependent kinase kinase. Nature Struct. Biol. 6, 819–824 (1999).
Schumacher, M. A., Rivard, A. F., Bachinger, H. P. & Adelman, J. P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410, 1120–1124 (2001).
Glaser, P. et al. Functional consequences of single amino acid substitutions in calmodulin-activated adenylate cyclase of Bordetella pertussis. EMBO J. 10, 1683–1688 (1991).
Munier, H. et al. The role of histidine 63 in the catalytic mechanism of Bordetella pertussis adenylate cyclase. J. Biol. Chem. 267, 9816–9820 (1992).
Tesmer, J. J. et al. Two-metal-Ion catalysis in adenylyl cyclase. Science 285, 756–760 (1999).
Tang, W. -J. & Hurley, J. H. Catalytic mechanism and regulation of mammalian adenylyl cyclases. Mol. Pharmacol. 54, 231–240 (1998).
Tesmer, J. J., Sunahara, R. K., Gilman, A. G. & Sprang, S. R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα-GTPγS. Science 278, 1907–1916 (1997).
Bieger, B. & Essen, L. O. Structural analysis of adenylate cyclases from Trypanosoma brucei in their monomeric state. EMBO J. 20, 433–445 (2001).
Galburt, E. A. et al. A novel endonuclease mechanism directly visualized for I-PpoI. Nature Struct. Biol. 6, 1096–1099 (1999).
Miller, M. D., Cai, J. & Krause, K. L. The active site of Serratia endonuclease contains a conserved magnesium-water cluster. J. Mol. Biol. 288, 975–987 (1999).
Yan, S. Z., Huang, Z. H., Shaw, R. S. & Tang, W. J. The conserved asparagine and arginine are essential for catalysis of mammalian adenylyl cyclase. J. Biol. Chem. 272, 12342–12349 (1997).
Lewit-Bentley, A. & Rety, S. EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 10, 637–643 (2000).
Zhang, M., Tanaka, T. & Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nature Struct. Biol. 2, 758–767 (1995).
Munier, H. et al. Structural characterization by nuclear magnetic resonance spectroscopy of a genetically engineered high-affinity calmodulin-binding peptide derived from Bordetella pertussis adenylate cyclase. Arch. Biochem. Biophys. 320, 224–235 (1995).
Glaser, P. et al. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 8, 967–972 (1989).
Labruyure, E. et al. Characterization of ATP and calmodulin-binding properties of a truncated form of Bacillus anthracis adenylate cyclase. Biochemistry 29, 4922–4928 (1990).
Rhoads, A. R. & Friedberg, F. Sequence motifs for calmodulin recognition. FASEB J. 11, 331–340 (1997).
Montgomery, H. J., Romanov, V. & Guillemette, J. G. Removal of a putative inhibitory element reduces the calcium-dependent calmodulin activation of neuronal nitric-oxide synthase. J. Biol. Chem. 275, 5052–5058 (2000).
Tesmer, J. J., Berman, D. M., Gilman, A. G. & Sprang, S. R. Structure of RGS4 bound to AlF-4-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell 89, 251–261 (1997).
Conte, L. L., Chothia, C. & Janin, J. The atomic structure of protein–protein recognition sites. J. Mol. Biol. 285, 2177–2198 (1999).
Meng, W., Sawasdikosol, S., Burakoff, S. J. & Eck, M. J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 398, 84–90 (1999).
Huang, X. et al. Structure of a WW domain containing fragment of dystrophin in complex with β-dystroglycan. Nature Struct. Biol. 7, 634–638 (2000).
Krueger, J. K. et al. Activation of myosin light chain kinase requires translocation of bound calmodulin. J. Biol. Chem. 276, 4535–4538 (2001).
Trewhella, J., Blumenthal, D. K., Rokop, S. E. & Seeger, P. A. Small-angle scattering studies show distinct conformations of calmodulin in its complexes with two peptides based on the regulatory domain of the catalytic subunit of phosphorylase kinase. Biochemistry 29, 9316–9324 (1990).
Farrar, Y. J., Lukas, T. J., Craig, T. A., Watterson, D. M. & Carlson, G. M. Features of calmodulin that are important in the activation of the catalytic subunit of phosphorylase kinase. J. Biol. Chem. 268, 4120–4125 (1993).
Hayashi, N., Izumi, Y., Titani, K. & Matsushima, N. The binding of myristoylated N-terminal nonapeptide from neuro-specific protein CAP-23/NAP-22 to calmodulin does not induce the globular structure observed for the calmodulin-nonmyristylated peptide complex. Protein Sci. 9, 1905–1913 (2000).
Wang, E., Zhuang, S., Kordowska, J., Grabarek, Z. & Wang, C. L. Calmodulin binds to caldesmon in an antiparallel manner. Biochemistry 36, 15026–15034 (1997).
Drum, C. L., Shen, Y., Rice, P. A., Bohm, A. & Tang, W. J. Crystallization and preliminary X-ray study of the edema factor exotoxin adenylyl cyclase domain from Bacillus anthracis in the presence of its activator, calmodulin. Acta Crystallogr. D 57, 1881–1884 (2001).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Cowtan, K. in Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography No. 31, 34–38 (1994).
Kissinger, C. R., Gehlhaar, D. K., Smith, B. A. & Bouzida, D. Molecular replacement by evolutionary search. Acta Crystallogr. D 57, 1474–1479 (2001).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47, 110–119 (1991).
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. 54, 905–921 (1998).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Esnouf, R. M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta Crystallogr. D 55, 938–940 (1999).
Acknowledgements
We thank Y. Mabuchi and A. Higbie for technical assistance; X. Yang for computer maintenance; B. Bernat for help with mass spectrometry; P. Gardner for DNA sequencing; J. Geiger, C. Harrison, G. Meinke, K. Moffat, J. Piccirilli, P. Rice, M. Rosner and C.-R. Wang for discussions; and the beamline personnel at NSLS beamline X25 and APS, especially those at BioCars. This work was supported by grants from the NIH NIGMS and American Heart Association to W.-J.T., by an NIH NIDA fellowship to C.L.D., by AHA fellowship to S.-Z.Y., and a grant from the NIH NIAMSD to Z.G. A.B. also acknowledges partial support from the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Drum, C., Yan, SZ., Bard, J. et al. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 415, 396–402 (2002). https://doi.org/10.1038/415396a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415396a
This article is cited by
-
Overexpression of the elongation factor MtEF1A1 promotes salt stress tolerance in Arabidopsis thaliana and Medicago truncatula
BMC Plant Biology (2023)
-
Chemical shift assignments of calmodulin under standard conditions at neutral pH
Biomolecular NMR Assignments (2022)
-
Calmodulin7: recent insights into emerging roles in plant development and stress
Plant Molecular Biology (2021)
-
Atomic structures of anthrax toxin protective antigen channels bound to partially unfolded lethal and edema factors
Nature Communications (2020)
-
Ca2+-dependent regulation of sodium channels NaV1.4 and NaV1.5 is controlled by the post-IQ motif
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.