Abstract
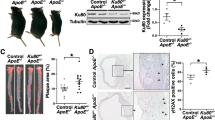
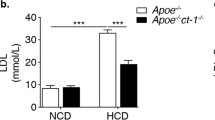
The tumor suppressor protein p53 is an essential molecule in cell proliferation and programmed cell death (apoptosis), and has been postulated to play a principal part in the development of atherosclerosis. We have examined the effect of p53 inactivation on atherogenesis in apoE-knockout mice, an animal model for atherosclerosis1,2. We found that, compared with p53+/+/apoE–/– mice, p53–/–/apoE–/– mice developed considerably accelerated aortic atherosclerosis in the presence of a similar serum cholesterol in response to a high-fat diet. Furthermore, the atherosclerotic lesions in p53–/–/apoE–/– mice had a significant (~280%) increase in cell proliferation rate and an insignificant (~180%) increase in apoptosis compared with those in p53+/+/apoE–/– mice. Our observations indicate that the role of p53 in atherosclerotic lesion development might be associated with its function in cell replication control, and that p53-independent mechanisms can mediate the apoptotic response in atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nakashima, Y. et al. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14, 133–140 (1994).
Reddick, R.L., Zhang, S.H. & Maeda, N. Atherosclerosis in mice lacking Apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 14, 141–147 (1994).
Virchow, R. Cellular Pathology as Based Upon Physiological and Pathological Histology (transl. Frank Chance, 1971) 1–554 (Dover, New York, 1863).
Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 (1993).
Geng, Y.-J. & Libby, P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1β-converting enzyme. Am. J. Pathol. 147, 251–266 (1995).
Isner, J.M., Kearney, M., Bortman, S. & Passeri, J. Apoptosis in human atherosclerosis and restenosis. Circulation 91, 2703–2711 (1995).
Han, D.K.M. et al. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am. J. Pathol. 147, 267–277 (1995).
Bjàrkerud, S. & Bjàrkerud, B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am. J. Pathol. 149, 367–380 (1996).
Kockx, M.M. Apoptosis in the atherosclerotic plaque. Arterioscler. Thromb. Vascular Biol. 18, 1519–1522 (1998).
Speir, E. et al. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265, 391–394 (1994).
Zhou, Y.F. et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherecomy. N. Engl. J. Med. 335, 624–630 (1996).
Donehower, L.A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Plump, A.S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992).
Donehower, L.A. Effects of p53 mutation on tumor progression: recent insights from mouse tumor models. Biochim. Biophys. Acta 1242, 171–176 (1996).
Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Rembold, C. Could atherosclerosis originate from defective smooth muscle cell death (apoptosis)? Persp. Biol. Med. 39, 405–408 (1996).
Bennett, M.R., Evan, G.I. & Schwartz, S.M. Apoptosis of rat vascular smooth muscle cells is regulated by p53-dependent and -independent pathways. Circ. Res. 77, 266–273 (1995).
Ko, L.J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Hundley, J.E. et al. Increased tumor proliferation and genomic instability without decreased apoptosis in MMTV-ras mice deficient in p53. Mol. Cell. Biol. 17, 723–731 (1997).
Jones, J.M. et al. Absence of p53 in a mouse mammary tumor model promotes tumor cell proliferation without affecting apoptosis. Cell Growth Differ. 8, 829–838 (1997).
Harvey, M. et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8, 2457–2467 (1993).
Palinski, W. et al. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Arterioscler. Thromb. 14, 605–616 (1994).
Conover, W.J. Practical Nonparametric Statistics 2nd ed. 213–343 (John Wiley & Sons, New York, 1980).
Acknowledgements
We thank M.E. DeBakey for his interest and support, L. Donehower for his advice, C. Langston for allowing us to use the video imaging system for these studies, E. Zsigmond for assistance with FPLC, M. Majesky for a critical reading of the paper and E. Boerwinkle of The Human Genetics Center of The University of Texas Health Science Center for assistance with the statistical analysis. This work was supported by an NIH grant (HL-51586).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guevara, N., Kim, HS., Antonova, E. et al. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med 5, 335–339 (1999). https://doi.org/10.1038/6585
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/6585
This article is cited by
-
TP53 clonal hematopoiesis promotes atherosclerosis
Nature Cardiovascular Research (2023)
-
TP53-mediated clonal hematopoiesis confers increased risk for incident atherosclerotic disease
Nature Cardiovascular Research (2023)
-
The regulatory roles of p53 in cardiovascular health and disease
Cellular and Molecular Life Sciences (2021)
-
Effect of cholesterol re-supplementation and atorvastatin on plaque composition in the thoracic aorta of New Zealand white rabbits
BMC Cardiovascular Disorders (2020)
-
Genetics of Arterial-Wall-Specific Mechanisms in Atherosclerosis: Focus on Mitochondrial Mutations
Current Atherosclerosis Reports (2020)