Abstract
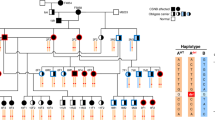
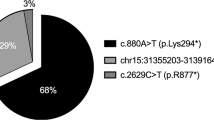
X-linked congenital stationary night blindness (CSNB) is a recessive non-progressive retinal disorder characterized by night blindness, decreased visual acuity, myopia, nystagmus and strabismus1,2,3. Two distinct clinical entities of X-linked CSNB have been proposed4. Patients with complete CSNB show moderate to severe myopia, undetectable rod function and a normal cone response, whereas patients with incomplete CSNB show moderate myopia to hyperopia and subnormal but measurable rod and cone function. The electrophysiological and psychophysical features of these clinical entities suggest a defect in retinal neurotransmission. The apparent clinical heterogeneity in X-linked CSNB reflects the recently described genetic heterogeneity in which the locus for complete CSNB (CSNB1) was mapped to Xp11.4, and the locus for incomplete CSNB (CSNB2) was refined within Xp11.23 (ref. 5). A novel retina-specific gene mapping to the CSNB2 minimal region was characterized and found to have similarity to voltage-gated L-type calcium channel α1-subunit genes. Mutation analysis of this new α1-subunit gene, CACNA1F , in 20 families with incomplete CSNB revealed six different mutations that are all predicted to cause premature protein truncation. These findings establish that loss-of-function mutations in CACNA1F cause incomplete CSNB, making this disorder an example of a human channelopathy of the retina.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Héon, E. & Musarella, M.A. Congenital stationary night blindness: a critical review for molecular approaches. in Molecular Genetics of Inherited Eye Disorders (eds Wright, A.F. & Jay, B.) 277–301 (Harwood Academic Publishers, London, 1994).
Pearce, W.G., Reedyk, M. & Coupland, S.G. Variable expressivity in X-linked congenital stationary night blindness. Can. J. Ophthalmol. 25, 3–10 (1990).
Carr, R.E. Congenital stationary night blindness. Trans. Am. Ophthalmol. Soc. 72, 448–487 (1974).
Miyake, Y., Yagasaki, K., Horiguchi, M., Kawase, Y. & Kanda, T. Congenital stationary night blindness with negative electroretinogram. Arch. Ophthalmol. 104, 1013–1020 (1986).
Boycott, K.M. et al. Evidence for genetic heterogeneity in X-linked congenital stationary night blindness. Am. J. Hum. Genet. 62, 865– 875 (1998).
Bech-Hansen, N.T., Boycott, K.M., Gratton, K.J., Ross, D. & Pearce, W.G. Localization of a gene for incomplete X-linked congenital stationary night blindness in Xp11.23 to the interval betweeen DXS6849 and DXS8023. Human Genet., (in press).
Boycott, K.M., Halley, G.R., Schlessinger, D. & Bech-Hansen, N.T. A 2-megabase physical contig incorporating 43 DNA markers on the human X chromosome at p11.23-p11.22 from ZNF21 to DXS255. Genomics 33, 488–497 (1996).
Schindelhauer, D. et al. Long-range map of a 3.5-Mb region in Xp11.23-22 with a sequence-ready map from a 1.1-Mb gene-rich interval. Genome Res. 6 , 1056–1069 (1996).
Boycott, K.M. et al. Construction of a 1.5 Mb bacterial artificial chromosome (BAC) contig in Xp11.23, a region of high gene content. Genomics 48, 369–372 (1998).
Strom, T. et al. An L-type calcium channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genet. 19, 260– 263 (1998).
Nathans, J. & Hogness, D.S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc. Natl Acad. Sci. USA 81, 193–202 ( 1984).
Bech-Hansen, N.T. & Pearce, W.G. Manifestations of X-linked congenital stationary night blindness in three daughters of an affected male: demonstration of homozygosity. Am. J. Hum. Genet. 52, 71–77 (1993).
Epp, F.H. Mennonites in Canada 1786-1920: The History of a Separate People (Macmillan Company of Canada, 1975).
Williams, M.E. et al. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 8, 71–84 ( 1992).
Stea, A., Wah, S.T. & Snutch, T.P. Voltage-gated calcium channels. in Ligand- and Voltage-Gated Ion Channels (ed. North, R.A.) 113–151 (CRC Press, Inc., Boca Raton, Florida, 1995).
Schuster, A. et al. The IVS6 segment of the L-type calcium channel is critical for the action of dihydropyridines and phenylalkylamines. EMBO J. 15, 2365–2370 (1996).
Fisher, S.E. et al. Sequence-based exon prediction around the synaptophysin locus reveals a gene-rich area containing novel genes in human proximal Xp. Genomics 45, 340–347 (1997).
Snutch, T.P. & Reniner, P.B. Ca2+ channels: diversity of form and function. Curr. Opin. Neurobiol. 2, 247–253 (1992).
Catterall, W.A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 64, 493–531 ( 1995).
Tanabe, T., Beam, K.G., Powell, J.A. & Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336, 134– 139 (1988).
Mikami, A. et al. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 340, 230– 233 (1989).
Doyle, J.L. & Stubbs, L. Ataxia, arrythmia and ion-channel gene defects. Trends Genet. 14, 92– 98 (1998).
Smith, L.A. et al. A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J. Neurosci. 16, 7868–7879 ( 1996).
Stockton, R.A. & Slaughter, M.M. B-wave of the electroretinogram: a reflection of bipolar cell activity. J. Gen. Physiol. 93, 101–122 (1989).
Sieving, P.A., Murayama, K. & Naarendorp, F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis. Neurosci. 11, 519–532 ( 1994).
Fishman, G.A. & Sokol, S. Electrophysiologic Testing in Disorders of the Retina, Optic Nerve, and Visual Pathway (Am. Acad. Ophthal., San Francisco, 1990).
Wilkinson, M.F. & Barnes, S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J. Gen. Physiol. 107, 621–630 (1996).
Yagi, T. & Macleish, P.R. Ionic conductances of monkey solitary cone inner segments. J. Neurophysiol. 71, 656–665 (1994).
Rieke, F. & Schwartz, E.A. A cGMP-gated current can control exocytosis at cone synapses. Neuron 13, 863–873 (1994).
Tachibana, M., Okada, T., Arimura, T., Koybayashi, K. & Piccolino, M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina . J. Neurosci. 13, 2898– 2090 (1993).
Boycott, K.M. et al. Integration of 101 DNA markers across human Xp11 using a panel of somatic cell hybrids. Cytogenet. Cell Genet. 76, 223–228 (1997).
Huang, X. On global sequence alignment. Computer Applic. Biosci. 10, 227–235 (1994).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 ( 1987).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 ( 1985).
Acknowledgements
We thank the participating families and especially the large Mennonite kindred that initiated our interest in identifying the CSNB2 gene. We also thank L. MacLaren, T. Mah, K. McElligott, M.L. Klimek and S. Scott for facilitating the collection of blood samples, and R. Winkfein, S. Barnes, P. Mains, D. Rancourt, W. Stell, M. Walter and G. Zamponi for helpful discussions. R. Winkfein and P. Schnetkamp provided us with first-strand cDNA from human retinal mRNA. This research was supported in part by the RP Research Foundation (Canada) (operating grants to N.T.B.-H. and Studentship to K.M.B.), the Canadian Genome Analysis and Technology Program and the I.D. Bebensee Foundation. N.T.B.-H. was supported by the Department of Ophthalmology (University of Alberta) and The Alberta Children's Hospital Foundation. K.M.B. is the recipient of an Alberta Heritage Foundation for Medical Research Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bech-Hansen, N., Naylor, M., Maybaum, T. et al. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness . Nat Genet 19, 264–267 (1998). https://doi.org/10.1038/947
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/947
This article is cited by
-
Structure, gating, and pharmacology of human CaV3.3 channel
Nature Communications (2022)
-
Biochemistry and physiology of zebrafish photoreceptors
Pflügers Archiv - European Journal of Physiology (2021)
-
Cav1.4 dysfunction and congenital stationary night blindness type 2
Pflügers Archiv - European Journal of Physiology (2021)
-
The role of voltage-gated ion channels in visual function and disease in mammalian photoreceptors
Pflügers Archiv - European Journal of Physiology (2021)
-
Submicromolar copper (II) ions stimulate transretinal signaling in the isolated retina from wild type but not from Cav2.3-deficient mice
BMC Ophthalmology (2020)