Abstract
Adipose tissue is not an inert cell mass contributing only to the storage of fat, but a sophisticated ensemble of cellular components with highly specialized and complex functions. In addition to managing the most important energy reserve of the body, it secretes a multitude of soluble proteins called adipokines, which have beneficial or, alternatively, deleterious effects on the homeostasis of the whole body. The expression of these adipokines is an integrated response to various signals received from many organs, which depends heavily on the integrity and physiological status of the adipose tissue. One of the main regulators of gene expression in fat is the transcription factor peroxisome proliferator-activated receptor γ (PPARγ), which is a fatty acid- and eicosanoid-dependent nuclear receptor that plays key roles in the development and maintenance of the adipose tissue. Furthermore, synthetic PPARγ agonists are therapeutic agents used in the treatment of type 2 diabetes.
This review discusses recent knowledge on the link between fat physiology and metabolic diseases, and the roles of PPARγ in this interplay via the regulation of lipid and glucose metabolism. Finally, we assess the putative benefits of targeting this nuclear receptor with still-to-be-identified highly selective PPARγ modulators.
Similar content being viewed by others
Introduction
In mammals, the ensemble of fat tissues constitutes a multi-depot adipose organ that is highly innervated and rich in blood vessels. It serves metabolic and endocrine functions, which are of critical importance for the integrative physiology of the body. This organ is not only composed of lipid-laden mature adipocytes and adipocyte precursors called preadipocytes, but also comprises a stromal vascular fraction (SVF), which includes blood cells, endothelial cells and macrophages. Although adipocytes have been recognized as secretory cells with endocrine functions for some time, the importance of macrophages and stromal vascular cells within the adipose tissue of obese animals and humans is now well accepted. This knowledge has contributed to a better understanding of the intense cross-talk between the different components of fat tissue, and has led to stimulating speculations about the initiation of pathological conditions. Furthermore, analysis of the sympathetic and parasympathetic innervations of adipose tissue revealed that the autonomic nervous system modulates the fat cell number and other processes, such as adipokine expression levels, lipogenesis/lipolysis, fatty acid uptake, and glucose uptake. These recent findings underscore the integrative role of the brain in long-term energy balance 1. As part of this interactive and integrated network, the adipose tissue per se is involved in the coordination of diverse processes including not only energy metabolism but also endocrine and immune regulatory functions. This review underlines the importance of the functional integrity of the adipose tissue in maintaining health. Both adipose tissue deficiency (lipodystrophy, lipoatrophy) and adipose tissue excess (obesity) have deleterious effects and constitute major medical problems and socioeconomic burdens all around the world today. Obesity, in particular, is associated with prothrombic and proinflammation states, hypertension, dyslipidemia, hyperglycemia, insulin resistance, degenerative diseases, and some cancers 2. The World Health Organization estimates that over 300 million people are clinically obese, and the dramatic increase in obesity among children underscores the urgent need for increased knowledge on adipocytes as regulators of energy balance, which will hopefully contribute to ameliorating the serious public health problem created by obesity.
Adipose tissue: the organ and its functions
The adipose tissue has two main functions. Firstly, it plays an important role in the storage and release of lipids 3, thus managing the energy reserve of the body according to supply and need. Secondly, it is a bona fide endocrine organ synthesizing and secreting a large variety of molecules called adipokines, which act both at the local (autocrine/paracrine) and systemic (endocrine) levels, and have an influence on all major organs involved in the physiology of the body 4, 5, 6.
There are several visceral (vis) and subcutaneous (sc) fat depots, each playing a specific role (Figure 1) 7. Some parts of these depots are predominantly white, and thus they form the white adipose tissue (WAT), while a few depots are predominantly brown, owing to a more dense irrigation and high numbers of mitochondria, and these correspond to the brown adipose tissue (BAT). WAT and BAT perform complementary functions in vivo. WAT essentially accumulates excess energy as fat and therefore constitutes the largest energy reservoir in mammals as a guard against times of food shortage. In contrast, BAT is highly specialized in non-shivering adaptive thermogenesis. Although the role of BAT in rodents and neonates of other mammalian species, including humans, has been extensively studied, the persistence and importance of BAT in adult humans is currently under intense investigation and putative functions remain to be elucidated 8, 9. In brief, the BAT and WAT closely collaborate in partitioning the energy contained in lipids between thermogenesis and other metabolic functions, respectively. This review will concentrate on the latter only.
Schematic representation of the human adipose organ, main adipose subtypes and functions. The rodent perigonadal fat, which has no relevance in humans, is depicted here only as a reminder of its broad use as an experimental model of vis fat. Different processes and adipokine expression are indicated with respect to their prevalence in sc WAT or vis WAT. * enhances insulin sensitivity; ** enhances insulin resistance.
WAT subtypes
Mature adipose cells, whose differentiation is controlled by a cascade of specific transcription factors, represent the major component of the adipose tissue. During the differentiation process, morphologically and functionally diverse tissues give birth to the sc and vis adipose tissues 4, 10, 11, 12, 13. Each of them has a different metabolic activity reflected in a different sensitivity to insulin 4, 14, 15. Different expression profiles in sc and vis WAT of several genes involved in embryonic development and pattern specification suggest different genetically determined developmental programs in preadipocytes for the formation of each depot with its specific functional characteristics 16. In addition to the main sc and vis depots, WAT is found in small amounts around other organs, such as the heart, kidney and genitalia.
Among these different tissues, the sc deposits are those that undergo the more conspicuous enlargements and retractions without noticeable effects on insulin sensitivity, glucose metabolism, and metabolic profile 17, 18. In humans, the sc adipose tissue can be subdivided into two distinct layers: the superficial and the deep layers. There is a gender dimorphism in the amount of deep layer sc WAT. Fifty-one percent of a woman's sc WAT is found in this layer, whereas in a man it comprises 66% of the sc WAT. It appears that obesity is associated with a preferential increase in the deep layer, and weight loss in obese people also impacts more on the deep layer, suggesting that the deep layer is metabolically more active than the superficial one 19.
The vis fat is found in both the intraperitoneal and retroperitoneal compartments. Intraperitoneal fat is itself composed of omental and mesenteric adipose tissues, and in rodents perigonadal (epididymal) adipose tissues, the latter being largely used as an experimental model for vis fat. The delimitation between the intraperitoneal and retroperitoneal fat is along the ventral surface of the kidney and the dorsal borderline of the intestines. In humans, the retroperitoneal fat is minor as it represents only 25% of the total vis part 15. Vis fat is distinct from other adipose regions since it is drained by the portal vein, and therefore has a unique direct connection with the liver. Reduction of vis deposits promotes insulin sensitivity and glucose metabolism. In fact, vis fat mass correlates positively with glucose intolerance, alteration of plasma lipoprotein lipid levels, increased triglyceride (TG) and cholesterol concentrations, hypertension, and dyslipidemia 20, 21. Moreover, insulin signaling analysis in human vis and sc fat shows that the vis adipose tissue expresses higher levels of specific insulin-signaling proteins and exhibits an earlier and greater response to insulin than the sc WAT 22. In short, vis fat is more affected by weight reduction than sc fat, is more active metabolically, has a higher lipolytic rate, and produces more adipokines (Figure 1) 15, 23.
These different characteristics of the two fat depots with respect to morphological structure, metabolic activity, and hormonal control suggest a specific role distribution between vis and sc WAT in whole-body energy homeostasis and a differential impact on insulin sensitivity in skeletal muscle and liver. These functions will not be discussed further since the information is already available in two reviews 15, 24.
WAT and energy homeostasis
One of the primary functions of WAT is to store excess energy as lipids, which are then mobilized to other tissues in response to metabolic needs during periods of food scarcity 9.
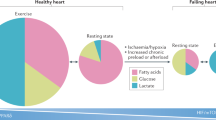
After copious meals, and in periods of food abundance, the adipose tissue stores the energy ingested in excess as TG (Figure 2A). The adipocyte is able to accumulate astonishingly high amounts of TG, which can be stored anhydrously within intracellular lipid droplets coated with proteins called perilipins, without causing cellular lipotoxicity 25, 26.
Role of the WAT and peripheral organs in post-prandial (A) and fasting (B) states. (A) In the post-prandial state, the ingested nutrients reach the circulation in the form of triglycerides (in chylomicrons as VLDL), glucose, and amino acids. In this state the main metabolic activities are the oxidation of glucose for the production of ATP in most cells, and the storage of excess fuel molecules in adipocytes as triglycerides, in hepatocytes as proteins, glycogen, and triglycerides, and in skeletal muscle fibers as proteins and glycogen. In fuel-excess conditions, amino-acid oxidation increases in proportion to increments in protein intake, regardless of carbohydrate and fat substrate availability. (B) Fasting induces breakdown of the triglycerides from WAT into free fatty acids and glycerol, both released into the circulation. Free fatty acids can be directly oxidized by skeletal muscle fibers for the production of ATP. Hepatocytes use glycerol for gluconeogenesis, and free fatty acids for ketone body synthesis. Both glucose and ketone bodies are important fuel molecules for the peripheral organs. In fuel shortage conditions, the extent to which amino-acid oxidation rises above the minimum required rate depends on the proportion of the energy needs, which is covered by FFA, glucose, and ketone body oxidation. Stored molecules (triglycerides, glycogen, proteins) are indicated in bold capitals. Adapted in part from 240.
Starvation induces the breakdown of these TG into free fatty acids (FFA) and glycerol, which are released into the circulation (Figure 2B). FFA then serve as fuels for metabolically active tissues such as the skeletal muscle where their oxidation to carbon dioxide and water generates ATP. In the liver, most of the acetyl CoA produced by FFA oxidation is used to synthesize ketone bodies (acetoacetate; b-hydroxybutyrate), which are released into the circulation and used as fuels by the peripheral tissues. The glycerol generated by TG hydrolysis serves for the synthesis of glucose, which is reserved for cells depending on it as an energy source (neurons, red blood cells), or participates in the hepatic production of TG 27. These TG are then packaged within very low-density lipoproteins (VLDL) and released into the circulation from where they can return to WAT. Regulation of the TG stocks is crucial for survival, since without WAT and its lipid reserve, animals would have to eat continuously, which is obviously not possible. For well-known reasons, food intake occurs only in distinct episodes underscoring the importance of a control of lipid production or intake, storage, and utilization.
Regulation of lipid metabolism in adipocytes is controlled at three levels: fatty acid uptake, lipogenesis, and lipolysis. Each of these processes is controlled by extracellular stimuli, including insulin, corticoids, catecholamines, natriuretic peptides, and cytokines such as TNF-α, whose levels depend on conditions such as age, gender, physical activity, and nutritional factors 28. In addition, there are marked differences between the vis and sc adipose tissues in the regulation and levels of lipid metabolic activities. For instance, nearly 80% of fat is in the sc tissue, but the lipolytic effect of catecholamines is more pronounced in the vis fat whereas the antilipolytic effect of insulin is stronger in the sc fat 21.
The adipose tissue participates in the regulation of glucose homeostasis, which depends on the action of other organs as well (pancreas, liver, brain). Firstly, it is involved in glucose disposal, which, via the glycolytic pathway, provides the substrate for the de novo synthesis of fatty acids and glycerol (and thus lipogenesis). Too little or too much adiposity is associated with hyperglycemia and insulin resistance. Not surprisingly, by liberating FFA into the circulation, the adipose tissue influences insulin sensitivity and thus glucose metabolism in the muscle and liver. Furthermore, among other functions, FFA serve as a substrate for lipoprotein assembly in the liver, regulate insulin production in the pancreas, and bind to and activate the transcription factors Peroxisome Proliferator-Activated Receptors (PPARs) in all tissues, which in turn results in gene expression changes and their consequences (see below) 10, 28, 29, 30. Thus WAT, by being involved in the regulation of lipid and glucose metabolism, not only in adipocytes but also in the whole body, plays an important integrative role in energy homeostasis especially in extreme conditions when food is available on a very irregular basis and/or is of variable nutritional quality.
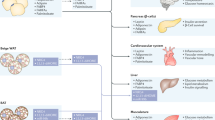
In mammals, when excess energy is not directed correctly into sc fat, it will preferentially accumulate in vis WAT, with deleterious effects, a condition often associated with a genetic susceptibility to vis fat obesity, and/or an endocrine-related maladaptive response to stress or smoking 31. When the adipose tissue is deficient, for instance because it is insulin resistant, or is abnormally distributed (lipodystrophy), the extra TG will be deposited ectopically in muscle, liver and heart, and to some extent also in the pancreas, which will be detrimental to the normal functioning of these now abnormally lipid-loaded tissues. This will impact on whole-body metabolism with the development of features of metabolic syndrome as a consequence (Figure 3) 31.
Obesity and lipodystrophy alterations of the WAT induce activation of the innate immune system, which is responsible for a low-grade inflammation of the tissue as well as an increased expression of several deleterious adipokines. Therefore, the plasma levels of adipokines, FFA, TG, and glucose are increased. Ectopic fat accumulation in the liver, skeletal muscle, or the pancreatic beta cells will alter their functioning and in consequence will affect the whole-body energy balance and promote the development of the metabolic syndrome.
WAT as an endocrine gland
For long regarded as a mere inert fat store for metabolic demands associated with fasting or exercise, WAT has finally emerged as a bona fide endocrine gland able to integrate hormonal signals from different parts of the body and respond by secreting its own signaling polypeptides called adipokines. These mediators have an impact on multiple target organs, such as the liver and skeletal muscle, and directly participate in the general control of the energy balance. Several of these adipokines, such as leptin and adiponectin, mediate some of their effect through activation of neuronal circuits in the hypothalamus and other brain areas with an impact on systemic regulation of energy expenditure and lipid catabolism 32. Besides the production of these adipokines, WAT, under stress conditions, also secretes pro- or anti-inflammatory cytokines, with autocrine and/or paracrine effector functions contributing to the control of energy homeostasis. Both the production and secretion of adipokines and pro- or anti-inflammatory cytokines by the WAT are regulated by the overall WAT mass (obesity or lipodystrophy) and the physiological status of the organism 33.
Only a few major adipokines will be considered further, namely those that have been implicated in the direct modulation of metabolism as well as pro/anti-inflammatory cytokines, which are produced under stress conditions and indirectly affect energy homeostasis.
Adipokines directly involved in energy homeostasis
As mentioned previously, adipocytes have a high capacity to produce and secrete adipokines, which act in an autocrine, paracrine or endocrine fashion to control several functions, including lipid and glucose metabolism, and insulin secretion. Deregulation of these processes contributes to the development of the metabolic syndrome. Although several adipokines involved in energy homeostasis were discovered in recent years, the so far most studied ones are leptin, adiponectin, resistin, angiopoietin-like protein 4, and preadipocyte factor 1, on which this section will concentrate. In addition, information on other adipokines is given in Table 1 and Figure 4, or can be found in several review articles 26, 34, 35, 36, 37, 38.
Leptin (from the Greek leptos, meaning thin) is a small 16 kDa polypeptide of 167 amino acids produced mainly by adipocytes, in direct proportion to adipose tissue mass. Its production is increased by estrogens, glucocorticoids, insulin, TNF-α, and C/EBPα, and decreased by PPARγ agonists, FFA, growth hormone, androgens, and β3-adrenergic activity. It is secreted in higher amounts by sc compared to vis fat (Figure 1). It reduces food intake through a direct effect on the hypothalamus 39. These observations contributed to viewing leptin as an antiobesity hormone, but it is now thought that it serves primarily as an energy sufficiency signal, whose levels decrease with weight loss or caloric restriction. Reduction in leptin levels during starvation is associated with adaptive responses including decreased energy expenditure and increased appetite. In several obesity models, the role of leptin is less obvious, since there was no improvement in spite of high endogenous leptin levels or treatment with exogenous leptin. Evidently, the target tissues have become resistant to leptin action, but the mechanism of this resistance remains unknown 40. However, hyperleptinemia induced by overnutrition prevents ectopic lipid deposition by acting on appetite via the hypothalamus, thereby limiting energy surplus storage in the available adipocytes. Furthermore, ectopic lipid deposition is minimized by increasing fatty acid oxidation and decreasing lipogenesis in peripheral tissues 41. In addition, the WAT itself has a leptin receptor-mediated energy regulating system, which is turned-off during overnutrition allowing the storage of excess calories and thereby diet-induced weight gain. This downregulation of the regulatory mechanism protects the whole organism against toxic ectopic lipid accumulation by permitting lipid accumulation in WAT 42. The organism appears most leptin sensitive in a range between low levels of the adipokine in situations of food restriction and its increasing levels during re-feeding, rather than in its supraphysiological concentrations such as those occurring in obesity 43. Exogenous leptin improves glucose homeostasis in ob/ob and lipodystrophic mice 44, 45. It also improves glucose homeostasis in humans with congenital leptin deficiency or lipodystrophy, but has little effect on classic obesity 46, 47, 48. In addition to its role in energy homeostasis, leptin has important endocrine functions, including the regulation of the hypothalamic-pituitary-adrenal and gonadal axes, bone development, immune response, angiogenesis, and hematopoiesis, which will not be reviewed here 49, 50.
Adiponectin, also referred to as AdipoQ or adipocyte complement related protein 30 (Acrp30, GBP28, apM1) 51 is a mature adipocyte-specific secretory protein with a molecular weight of approximately 30 kDa, which shares homology with complement C1q, and types VIII and X collagen. Its expression is higher in sc than in vis fat (Figure 1), and post-translational hydroxylation and glycosylation produce multiple isoforms. It circulates in serum at high concentrations (several micrograms per ml) as a hexamer of relatively low molecular weight and a larger multimeric structure of high molecular weight (12-18 subunits). Its biological effects depend both on the circulating concentrations and properties of the different isoforms, and on the tissue-specific expression of the two adiponectin receptors (AdipoR1 and AdipoR2) distantly related to the G protein-coupled receptor 51. In some cells, T-cadherin may function as a co-receptor to transmit adiponectin metabolic signals 52. Experimental data suggest that adiponectin has antidiabetic, anti-inflammatory and antiatherogenic effects 37, 53. Only the former are summarized here, since they play important roles in energy homeostasis, obesity, and insulin sensitivity. In mice, decreased adiponectin is involved in the development of insulin resistance in models of both obesity and lipoatrophy 54. Adiponectin stimulates phosphorylation and activation of the 5′-AMP-activated protein kinase in skeletal muscle and the liver, thereby regulating insulin sensitivity and glucose metabolism 55. In muscle, adiponectin stimulates fatty acid oxidation and glucose catabolism, and in the liver it reduces glucose output and FFA influx, and increases fatty acid oxidation and insulin sensitivity. In Rhesus monkeys, plasma levels of adiponectin were shown to decrease parallel with reduced insulin sensitivity even before the onset of type 2 diabetes 56, and in Japanese men with type 2 diabetes, hypoadiponectinemia was found to be associated with vis fat accumulation and insulin resistance 57. In contrast, an increase in adiponectin levels is observed after administration of thiazolidinediones (TZDs), angiotensin-converting enzyme inhibitors, and the angiotensin II receptor blocker (ARB), after weight loss, renal failure, heart failure, or after intake of soy protein or oils 51. Other effects of adiponectin on monocytes/macrophages, angiogenesis, and nitric oxide production are not discussed here.
Resistin (resistance to insulin, also called FIZZ3 or ADSF) is another small protein (12.5 kDa) and a member of the hormone family of cysteine-rich resistin-like molecules 58, which is mainly produced and secreted by WAT and appears to increase glucose production in the liver by a specific insulin antagonizing action 59. In rodents, it is expressed 15 times more in vis compared to sc fat (Figure 1) and it is highly upregulated in models of diet-induced obesity, as well as in genetic models of obesity and diabetes. Resistin circulates in multimeric forms, probably corresponding to trimers and hexamers, the conversion of the latter to the trimeric form most likely representing an obligatory step towards activation 60. Mice treated with recombinant resistin develop insulin resistance and glucose intolerance, while cultured adipocytes receiving the same treatment are impaired in insulin-stimulated glucose uptake, suggesting that resistin affects insulin action. Resistin-null mice have similar fat mass and weight as wild-type (WT) mice and show improved glucose homeostasis associated with a decrease in hepatic gluconeogenesis 59, 61, 62. In addition, resistin appears to be a pro-inflammatory cytokine. There are substantial inter-species differences between the sites of resistin production, occurring, for example, in adipocytes in rodents, and most likely non-fat cells, macrophages or other stromal cells present in the adipose tissue in humans 63, 64, 65, 66. The role of resistin in humans is not well established, since clinical studies do not show a clear link between obesity and insulin resistance versus resistin levels 67, 68.
Angiopoietin-like proteins (ANGPTLs) are also involved in the regulation of energy homeostasis 69. One of them, the fasting-induced adipose factor (FIAF), also called ANGPTL4, HFARP and PGAR 70, 71, 72, 73, is most highly expressed in the adipose tissue and secreted into the circulation, where it is found associated with plasma lipoproteins. Adenovirus-mediated overexpression of FIAF was found to improve hyperinsulinemia, hyperglycemia and glucose tolerance 74. Yet it is a powerful signaling molecule that inhibits plasma TG clearance and thus fat storage, and promotes TG mobilization by stimulating adipose tissue lipolysis possibly by the inhibition of lipoprotein lipase activity 75. Thus, FIAF is efficient in both reducing blood glucose and improving insulin sensitivity, while simultaneously promoting hyperlipidemia and fatty liver. Although much remains to be discovered about this intriguing dual function of FIAF, one can speculate that it connects adipose tissue to the modulation of the levels of plasma lipids. Modification of FIAF production and signaling may contribute to the development of dyslipidemia and possibly type 2 diabetes 75.
Lastly, proteolytic cleavage of the transmembrane protein preadipocyte factor 1 (Pref-1), highly expressed in preadipocytes, but not in adipocytes where it plays the role of an inhibitor of adipogenesis, gives birth to two soluble proteins of 50 and 25 kDa. In transgenic mice, overexpression of Pref-1 specifically in WAT reduces the expression of adipocyte proteins such as leptin and adiponectin, and produces substantial loss of WAT, while these mice suffer from hypertriglycemia, impaired glucose tolerance and decreased insulin sensitivity. These findings demonstrate that Pref-1-induced impairment of adipocyte functions in vivo leads to the development of metabolic abnormalities 76.
Adipokines, chemokines, and vascular proteins involved in pro/anti-inflammatory reactions
Several molecules involved in inflammatory processes are produced by the adipose tissue in situations of stress, such as in obesity or lipodystrophy. Some of these adipokines are synthesized by adipocytes, whereas others are produced by the haematopoietic cell fraction of the WAT (in macrophages, T-cells, B-cells, natural killer cells), or cells from the SVF 36, 77, 78. A large number of studies suggest that adipose tissue inflammation is associated with metabolic diseases, such as insulin resistance and other obesity-related complications, and lipodystrophy 79, 80, 81, 82, 83, 84 (reviewed in 85). Most of the better-defined adipokines, which are involved in inflammation, are discussed briefly in this section, while a more exhaustive list is given in Table 2 and Figure 4.
The plasmatic levels of TNF-α, a 26-kDa transmembrane protein that is cleaved to a 17-kDa biologically active polypeptide, are relatively low in general. Within WAT, it is expressed in adipocytes and stromovascular cells at higher levels in sc compared to vis adipose tissue (Figure 1). However, the TNF-α produced by the adipocytes has only a local effect since it cannot be secreted. Thus, it is the macrophage-produced TNF-α that is responsible for the systemic effects 86. Although there is no clear correlation between obesity and insulin resistance versus levels of plasma TNF-α, expression of this cytokine in WAT correlates with these two pathologies 87, 88. Chronic exposure of mice to TNF-α induces insulin resistance, decreases the expression of genes involved in adipogenesis and lipogenesis in WAT, and promotes lipolysis, while deletion of the TNF-α gene improves circulating FFA and insulin sensitivity in mouse obesity 89. Besides its role in WAT, TNF-α increases the expression of genes involved in the de novo synthesis of FA, and decreases that of genes involved in FA oxidation in liver. The autocrine and paracrine effects of TNF-α are responsible for the insulin resistance observed in humans and rats to whom this cytokine has been administrated 90, 91, 92. TNF-α also regulates the expression of other adipokines in WAT. For instance, it downregulates the expression of adiponectin and increases the expression of IL-6, another cytokine involved in the endocrine role of WAT (see below) 93.
IL-6 is found at high levels in the plasma in multiple glycosylated forms ranging from 22 to 27 kDa, a third of which is produced by adipocytes. Its synthesis and secretion are approximately three times greater in vis compared to sc adipose tissue. The plasma levels of IL-6 positively correlate with fat mass, obesity, impaired glucose tolerance and insulin resistance, and thus could be used to predict the development of type 2 diabetes and cardiovascular diseases 6, 94. IL-6, like TNF-α, modulates the insulin sensitivity of the liver and of skeletal muscle, thereby supporting the notion that cytokines produced by the adipose tissue influence whole-body insulin sensitivity. It is thought that IL-6 increases the expression of Socs-3 (inducing-suppressor of cytokine signaling-3) that negatively regulates insulin and leptin signaling. Central administration of this cytokine in rodents decreases body fat by increasing energy expenditure. In line with this effect, transgenic mice overexpressing IL-6 have reduced fat pad and body weights, which are associated with a growth defect 95, 96, 97.
In humans, the circulating levels of TGF-β1, a homodimer composed of two 12.5-kDa subunits, correlate positively with the body mass index (BMI) 98. This correlation also exists between the BMI and TGF-β1 production in WAT, a feature that holds especially for the vis WAT that produces more than two-fold more TGF-β1 compared to the sc WAT in humans 99, 100. However, the adipocytes are not the main producers of WAT TGF-β1, as the non-fat cells in the adipose tissue release more than 90% of it, production of which can be inhibited by TNF-α as well as by IL-1 99. In addition to its association with adipogenesis, TGF-β1 has multiple functions in a wide range of tissues, which will not be discussed here.
Mature monocytes produce monocyte chemotactic protein 1 (MCP1), a non-glycosylated 76 amino-acid polypeptide (8.7 kDa) usually found at low levels. IL-1, TNF-α and LPS rapidly induce its expression and secretion. MCP1 expression in WAT and MCP1 circulating levels are increased in obesity (rodents and humans). Conversely, its circulating levels are reduced parallel to weight loss 101. In vis WAT especially, MCP1 is not only produced by the infiltrated macrophages but also by adipocytes 102. In adipocytes, MCP1 influences lipid metabolism by downregulating PPARγ, which regulates lipid accumulation in these cells (the roles of PPARγ will be further discussed later). Moreover, MCP1 also stimulates leptin secretion and decreases insulin-stimulated glucose uptake in adipocytes. Transgenic animals overexpressing MCP1 in WAT only develop a normal adipose depot mass, but the tissue is infiltrated by an increased number of macrophages and produces elevated amounts of TNF-α and IL-6. Furthermore, the plasmatic levels of FFA in these animals are increased, most likely reflecting an increased lipolysis. In addition, the overexpression of MCP1 in WAT influences insulin sensitivity in the liver and especially in skeletal muscle, where it disturbs the insulin signaling pathway 51, 86, 103. Consistently, these mice become insulin resistant and glucose intolerant. These observations indicate that deregulation of cytokine expression in WAT can affect the overall metabolism of the body, particularly its insulin sensitivity 103, 104. Furthermore, it suggests that MCP1-mediated macrophage infiltration of fat might contribute to metabolic deregulations associated with insulin resistance and obesity. In rodent obesity, increased circulating levels of MCP1 positively correlate with increased monocytes, a phenotype also seen after peripheral administration of MCP1 to mice. In these animals, accumulation of monocytes in collateral arteries and enhanced neointimal formation might implicate MCP1 in the development of atherosclerosis.
At least two proteins, plasminogen activator inhibitor-1 (PAI1, 45 kDa) and tissue factor (TF, 47 kDa), which are involved in the fibrinolytic system and vascular hemostasis, are secreted by the WAT. PAI1 is a serine protease inhibitor protein (serpin), that is the principal inhibitor of tissue plasminogen activator and urokinase, which activate plasminogen and hence cause the physiological breakdown of blood clots (fribrinolysis). This protein is expressed and secreted in the WAT of rodents (higher in vis compared to sc adipose tissue) and humans, where its expression is regulated by TNF-α and TGF-β1, themselves produced by WAT 100, 105. The circulating levels of PAI1 are correlated with obesity and insulin resistance, and thus predict future risk of type 2 diabetes and cardiovascular disease 106, 107. The adipose tissue is thought to be an important contributor to the elevated plasmatic PAI1 concentrations in obesity 108, 109, but the mechanisms underlying the association between PAI1 levels and the disturbances found in the metabolic syndrome are not well understood. However, inhibition of fibrinolysis by PAI1 might be responsible for the high incidence of cardiovascular diseases, which is a feature of this syndrome 110.
TF is a protein released from damaged tissue that triggers the clotting cascade. It acts as a cell-surface receptor for the activation of factor VII. Its expression is upregulated in the WAT of ob/ob mice 111. Besides its role in coagulation, TF is thought to be involved in vascular development and integrity 112. In obesity, where the adipose mass is greatly increased, the need for oxygen supply is dramatically augmented. In this condition, it is likely that TF is involved in the angiogenesis associated with fat mass expansion.
Several proteins of the classic renin angiotensin system (RAS) are synthesized in the WAT. Adipose tissue RAS is considered to be a potential link between hypertension and obesity. The intimate relationship between WAT and RAS may also have a role in the pathophysiology of type 2 diabetes, especially in obese individuals. These points have been addressed in different review articles 43, 113, 114. Finally, the adipose tissue also expresses a variety of enzymes implicated in the activation, inter-conversion, and inactivation of steroid hormones that are also involved in the regulation of metabolic pathways. This role will not be discussed here as it has been reviewed recently 115. Similarly, the role of adipokine in the interaction between adipose tissue and immunity has been summarized recently and will not be presented here 34, 77.
Pathologies of the WAT
From the above, it has already become obvious that balanced amounts of adipose tissue are critical for an optimal regulation of lipid and glucose metabolism. Excess adiposity contributes to the development of insulin resistance, dyslipidemia, inflammation, hypertension, and cardiovascular diseases, while selective loss of WAT, called lipodystrophy, also predisposes to the same complications (Figure 3). Both branches of these deregulations, obesity and lipodystrophy, are addressed below.
Obesity
When the energy balance is positive, as often occurs with western diet, the adipose tissue becomes hypertrophic and subsequently hyperplastic. Since the adipocytes cannot expand beyond a “critical size”, which is thought to be genetically established for each depot type, the adipocyte number is increased when this critical point is reached 116, 117. Combined together, cell size and cell number increases lead to an expansion of the adipose tissue, which ultimately results in obesity 7.
Obesity produces what is called a “low-grade” inflammatory reaction in the adipose tissue by a mechanism that remains largely unknown 118. As presented in a former section, autocrine, paracrine, and endocrine signals from adipocytes, together with increased adipose tissue mass stimulate the synthesis and secretion of adipokines that trigger macrophage infiltration in the WAT. As already mentioned, there is a positive correlation between the adipocyte size and BMI, and the increase in adipokine expression in WAT 101, 119, 120, 121, 122. This low-grade inflammation is thought to result from a chronic activation of the innate immune system 83. The involvement of obesity in this process was suggested by the decrease of inflammation in the WAT of obese patients after weight loss 101, 123. Low-grade inflammation in WAT impairs its ability to control plasmatic FFA, promotes its deleterious endocrine function, and ultimately leads to insulin resistance, impaired glucose tolerance, and may result in diabetes and cardiovascular diseases (Figure 3).
It was shown in models of obese rodents as well as in humans that obesity is linked to an increase in adipocyte size. This hyperplasia is associated with an increased number of necrotic-like dead adipocytes surrounded by infiltrated macrophages, and the progressive up-regulation of inflammatory genes, such as TNF-α within the WAT. Moreover, this up-regulation precedes the dramatic increase in the circulating insulin levels, suggesting that the inflammatory reaction in the WAT is responsible for systemic insulin resistance. In addition, the persistence of WAT inflammation is responsible for the maintenance of insulin resistance in obese models 84, 86, 121, 124, 125, 126.
The genetics of human obesity unveiled the key role of leptin and melanocortin pathways, but only in rare cases. In fact, it is more a myriad of polymorphisms in genes and candidate regions, which defines the susceptibility of an individual to weight gain, a susceptibility that is accentuated by a permissive environment (diet, sedentarity) 127.
Lipodystrophy
Lipodystrophies are characterized by the absence of fat store development, the altered distribution of these reserves or their loss with, as a consequence, an excess accumulation of lipids in the liver, skeletal muscle and other organs, along with the emergence of insulin resistance (Figure 3) 128. It was demonstrated recently in mice that the membrane-anchored metalloproteinase MT1-MMP is required for WAT development and function. In its absence, the animals are lipodystrophic. MT1-MMP governs the interaction between the adipocyte and the extracellular matrix, and hence acts as a three-dimensional-specific adipogenic factor 129.
The different human lipodystrophic syndromes are defined by an altered quantity and/or distribution of adipose tissue (lipoatrophic peripheral sc fat and increased vis fat). In humans, the classification of lipodystrophies is usually made according to their origin, either genetic or acquired. Among the inherited lipodistrophies, some are better characterized, among which are the familial partial lipodystrophy Dunnigan-type (FPLD) and the familial generalized lipoatrophy known as Berardinelli-Seip congenital lipodystrophy (BSCL). FPLD is characterized by a loss of sc fat, while the inter- and intra-muscular fats as well as the abdominal fat are preserved. In adult patients, insulin resistance and type 2 diabetes correlate with an increase in plasma TG and FFA concentrations as well as the presence of C-reactive protein. Several FPLD patients also suffer from dyslipidemia and hypertension. In 50% of the FPLD families, there is a link between FPLD and the LMNA (lamin A) gene also associated with premature forms of aging, which codes for the nuclear envelope protein lamin A/C 130. Different mutations in this gene have been identified as culprits for lipodystrophy, but the mechanism by which it occurs is not known 131, 132, 133. Mutations in the LMNA gene are linked to a decrease in the plasma concentrations of adiponectin and leptin, and an increase in circulating TNF-α concentrations, which may cause the insulin resistance observed in FPLD patients 134. Another gene involved in FPLD encodes PPARγ, a transcription factor involved in adipogenesis.
BSCL is a generalized lipoatrophy characterized by the total loss of WAT, which is associated with insulin resistance and increased plasma TG levels. It is caused by mutations in two genes independently linked to this pathology. These genes encode seipin, a protein of unknown function, and 1-acylglycerol-3-phosphate-acyl transferase, which is involved in TG synthesis 135, 136.
Among the acquired lipodystrophies, the most common is the one associated with the antiretroviral treatments in HIV-infected patients. Fifty percent of these patients suffer from lipoatrophy, often associated with dyslipidemia, impaired glucose tolerance and diabetes. Interestingly, treatment of the patients with protease inhibitors (which are a part of the pharmacopoeia of HIV treatment) markedly alters the expression and secretion of adipokines from WAT. While adiponectin expression and secretion are decreased, IL-6 and TNF-α expression is upregulated in these patients. In WAT, increase in the production of these inflammatory cytokines is correlated to a decrease in adipocyte size, an increase in fibrosis, and infiltration of macrophages 137, 138, 139, 140.
Altered metabolism due to the loss of WAT is also observed in mouse models of lipodystrophy. Induced fat-cell apoptosis through targeted activation of caspase 8 causes WAT distrophy, glucose intolerance, and signs of inflammation 81. The same phenotype of adipocyte death, WAT fibrosis, macrophage infiltration, and increased inflammation is seen in mice with PPARγ specifically deleted from WAT in adult animals (see below) 141.
In conclusion, the study of lipodystrophy resulting from an impaired development of body fat or, alternatively, its altered distribution revealed a link between this pathology and deregulation of lipid and glucose metabolism with insulin resistance. Therefore, lipodystrophic patients, independent of the origin of lipodystrophy, either genetic or acquired, suffer from major complications with a prevalence of diabetes, cardiovascular diseases, pancreatitis, and liver steatosis with an evolution towards cirrhosis. Treatments with hypoglycemic and hypolipidemic drugs are beneficial therapeutic options for these patients, and for those with very low leptin levels, leptin treatment provides a major improvement 142, 143.
Role of the PPARγ in WAT
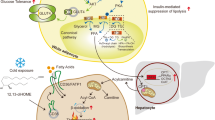
Adipocyte differentiation is intimately associated with the pathologies linked to WAT such as obesity, lipodystrophy, and inflammation, as described above. PPARs compose a subgroup of three receptors, belonging to the nuclear hormone receptor family, and acting as lipid sensors to modulate gene expression 144, 145. They are implicated in both major metabolic regulations and processes controlling cellular fate 146. In this part of the review, we will concentrate on one of the three PPAR isotypes, PPARγ, which is a pivotal coordinator of adipocyte differentiation and fatty acid uptake and storage (Figure 5).
PPARγ and adipocyte differentiation at the cellular level
Both adipocyte number and adipocyte size are major contributors to adipose tissue mass. Therefore, adipocyte differentiation is crucial in the maintenance of adipose tissue integrity. Adipocytes are either derived from resident differentiated preadipocytes or from progenitor cells 147, 148. PPARγ is a key player in this process (Figure 5). It has been recently shown that activated PPARγ not only stimulates differentiation to adipocytes of resident adipose tissue preadipocytes but also promotes the mobilization of bone marrow-derived circulating progenitor cells to WAT and their subsequent differentiation into adipocytes 149, 150. These results add an unexpected dimension to the field since they demonstrate, for the first time, that cells that reside outside the adipose tissue can influence and contribute to its fate.
Alternative promoter usage gives rise to two PPARγ isoforms. PPARγ1 is the ubiquitous isoform found in all PPARγ-expressing tissues such as WAT, BAT, macrophages, liver, skeletal muscle, kidney, colon, vascular endothelium and others 151, 152. PPARγ2 has a 30 (rodents) or 28 (human) residue N-terminal extension over that of PPARγ1, and is expressed primarily in adipose tissue 153, 154. The role of PPARγ1 and PPARγ2 as key regulators of adipocyte differentiation from preadipocytes was shown by several groups 154, 155, 156. Based on cellular studies, the differentiation of preadipocytes to adipocytes can be divided into four steps. First, the preadipocytes are withdrawn from the cell cycle, and genes responsible for the “preadipocyte phenotype” are downregulated. The second step, called the “mitotic clonal expansion”, allows a last round of cell division. Next, 48 h after the initiation of differentiation, the cells start to acquire the “early adipocyte phenotype”, which represents the third step. Fourth, in the “differentiated adipocytes”, genes already expressed at low levels in the early adipocyte phenotype, are now at their maximal expression levels, especially genes involved in energy storage and fat metabolism, such as C/EBPβ and PPARγ 157, 158. 3T3L1 cells, frequently used as adipocyte differentiation models, which have been manipulated to express small interference RNA (siRNA) against PPARγ or embryonic stem cells (ES cells) deficient for PPARγ (PPARγ−/−), are unable to differentiate into adipocytes. These defects in PPARγ expression reveal the important involvement of the receptor in this differentiation process, especially in the transition between the “mitotic clonal expansion” and the acquisition of the “early adipocyte phenotype” 159, 160. Conversely, experiments on gain of function, using retroviral expression of PPARγ in cultured fibroblasts, as well as treatment of fibroblasts with PPARγ agonists, were shown to stimulate adipogenesis 161.
Obviously, PPARγ is not the only transcription factor controlling the differentiation of mesenchymal cells to adipocytes, but a major player in a sophisticated network of transcription factors and their co-repressors and co-activators, which respond to specific stimuli to repress or stimulate adipocyte formation. The elegant cascade of transcription factor signaling in the regulation of adiposeness has been reviewed recently 162, and therefore will not be discussed further here.
PPARγ and lipid storage
In addition to being involved in the differentiation of adipocytes, PPARγ participates in the function of the mature cells. Indeed, PPARγ is the major regulator of lipid storage in WAT 163. It promotes the release of FFA from circulating lipoproteins by regulating lipoprotein lipase expression 164, and stimulates their uptake by enhancing the expression of the fatty acid translocase CD36, and of the fatty acid transport protein FATP1 165, 166, 167. Furthermore, PPARγ regulates the intracellular retention/transport of FFA by controlling the expression of fatty acid binding proteins 168. It also promotes the esterification of FFA into TG and their storage by regulating the expression of enzymes such as phosphoenol pyruvate carboxykinase, glycerol phosphate dehydrogenase, and diacylglycerol O acyltransferase. Expression of perilipin, which is the predominant protein associated with adipocyte lipid droplets and has a key function in regulating adipocyte lipid storage and body fat accumulation, is stimulated, too 167, 169, 170, 171, 172, 173, 174. Finally, PPARγ participates in the de novo FFA synthesis by regulating directly or indirectly the expression of enzymes such as fatty acid synthase, acetyl CoA synthetase, and stearoyl CoA desaturase 1 (Figure 5) 163, 166, 167, 175.
Ablation of PPARγ expression and activity in WAT: lessons from mouse models and human genetics
Studies on the role of PPARγ in WAT have been stimulated by the finding that TZDs, now used to treat patients suffering from type 2 diabetes, are specific ligands of PPARγ. Most of the present knowledge on PPARγ functions in energy homeostasis and its deregulations derives from the use of animal models and the investigation of patients bearing variant forms of the PPARγ gene (Figure 5).
Mouse models
General ablation of the PPARγ gene in mice is lethal due to placental malformation 176. In a model of generalized PPARγ ablation where embryonic lethality is prevented by preserving PPARγ expression in trophoblasts, severe lipodystrophy, insulin resistance and hypotension, probably due to increased vascular relaxation, were observed 177. On the contrary, the PPARγ+/− heterozygous animals are viable and do not present any major defects except mild growth retardation in males, possibly due to a deregulation of growth hormone signaling in the WAT 178. The PPARγ+/− mice have normal insulin sensitivity under a standard diet. However, when on a high-fat diet (HFD) and compared to WT animals, they are protected against fat mass increase, which is reflected in smaller adipocytes. Furthermore, they do not develop insulin resistance or liver steatosis, and display a substantial increase in FA oxidation in the liver and in skeletal muscle 179, 180, 181.
Specific deletion of PPARγ in WAT has led to a better understanding of pathologies linked directly to PPARγ dysfunction in this tissue. Three different laboratories performed this specific genetic manipulation. In a first study, using mutant animals on a standard diet, adipocyte hypocellularity and hypertrophy were observed, involving an increase in the levels of plasma TG and FFA, and a decrease in leptin and adiponectin levels, which were accompanied by increased hepatic gluconeogenesis and insulin resistance. This latter was reversed by TZD treatment that, however, failed to lower circulating FFA. These animals were more susceptible to HFD-induced steatosis, hyperinsulinemia and associated insulin resistance 182. A similar phenotype was observed in a “knock in” mouse model using the dominant-negative mutant PPARγL466A, which again showed the relationship between PPARγ function, adipose tissue and typical metabolic syndrome pathologies. Homozygous PPARγL466A mice died in utero, similar to PPARγ−/− mice 183.
In a second ablation study, mutant mice lacking PPARγ in adipose tissue were fed HFD, following which they presented diminished weight gain and plasma levels of adiponectin and leptin, but, in contrast to the first study, did not develop systemic insulin resistance or glucose intolerance. Furthermore, the mice exhibited diminished glucose uptake in the skeletal muscle, which suggests insulin resistance in this tissue. However, the liver did compensate for this insulin resistance by increasing glucose uptake and utilization, thereby improving the overall systemic insulin sensitivity. This improvement coincided with an increased expression of PPARγ in the liver, where it might have had a protective effect under these conditions 184. The reason for the difference in insulin resistance between the two studies remains unclear, but different feeding protocols might be the cause. These studies also showed that several genes involved in lipid uptake and lipogenesis were downregulated. The resulting diminution of fat accumulation in the WAT of these animals most likely contributed to the plasmatic increase in FFA and TG concentrations as well as to hepatic steatosis. 182, 184.
In the third study, ablation of PPARγ was induced selectively in adipocytes after the animals had reached adulthood. PPARγ-null adipocytes died within a few days after ablation of the gene, thus demonstrating that, in addition to its role in adipose differentiation, PPARγ is essential for the survival of mature adipocytes 141. In the studies discussed above, both isoforms PPARγ1 and PPARγ2 were deleted. When PPARγ2 alone is selectively disrupted, the mutant mice develop normally and are viable. However, they display a reduced WAT mass with smaller and heterogeneous-in-size adipocytes reflecting less lipid accumulation, well in line with a decreased expression of lipogenic genes. However, there was no liver steatosis, and insulin resistance was observed in male mice only. It was corrected by TZD treatment, probably by the effect of this drug on the remaining PPARγ1 in WAT, liver, and skeletal muscle. This model underscores again that the integrity of the adipose tissue is primordial for a good whole-body energy balance as well as for systemic insulin sensitivity. In addition, it shows that PPARγ1 alone can sustain development in general and drive adipose tissue formation in particular 185.
Another model in which the expression of PPARγ2 and γ1 is blunted in WAT, without affecting PPARγ1 expression in the liver and skeletal muscle, but in which PPARγ1 was found to increase in BAT, was called the PPARγ hypomorphic mouse (PPARγhyp/hyp) 186. PPAR hyp/hyp mice present a severe lipodystrophic syndrome and a relatively high neonatal mortality. Even if the surviving mice develop hyperlipidemia, they present only limited metabolic consequences of the severe WAT lipodistrophy most likely because of compensation, particularly by muscles 186.
The site of action of TZDs has been long debated and although not fully clarified yet, significant knowledge has come from the use of the A-ZIP/F1 fatless mouse that lacks WAT. These mice present a phenotype similar to that of humans with lipoatrophic diabetes, fatty liver, hyperlipidemia, and hyperglycemia and insulin resistance. Treatment of these animals with rosiglitazone and troglitazone (two PPARγ agonists; see “PPARγ as a therapeutic target in fat-related diseases”) showed that adipose tissue is required for the antidiabetic, but not for the hypolipidemic effect of TZDs 187. Using the same model, it was shown that rosiglitazone enhances insulin action in skeletal muscle by the distribution of fat away from this organ, contributing at least in part to liver steatosis. Ablation of liver PPARγ in the A-ZIP/F1 mice, while reducing steatosis, aggravates TG clearance problem, hyperlipidemia and, as a consequence, muscle insulin resistance 188, 189.
The results obtained from these different mouse models underscore the link between adipogenesis and the metabolic syndrome 190, and highlight the crucial role of PPARγ for the development, integrity and well-functioning of the WAT. Adipocytes communicate with preadipocytes, monocytes/macrophages and endothelial cells within the adipose tissue and with the liver, skeletal muscle, pancreas and brain at the systemic level. Most importantly, it shows that deregulation of the WAT function and integrity, which often interferes with the production of secreted adipokines and other signaling proteins by the different cell types comprised in the WAT, ultimately affects the homeostasis of the whole body. A disturbance of this balance contributes to the development of the metabolic syndrome and associated risks 31.
Human genetic studies
As highlighted by the animal models, PPARγ is a determining factor for fat-related pathologies. Similarly, arrays of polymorphisms and mutations have been identified in the human PPARγ gene, which are linked to metabolic phenotypes. Only mutations particularly informative on the role of PPARγ in WAT will be discussed below, with regard to adipose mass (obesity and lipodystrophy), energy balance, insulin resistance, and low-grade inflammation.
PPARγ loss of function mutations
Familial partial lipodystrophy (FPL) is associated with mutations in the PPARγ gene in a few patients. This partial lipodystrophy affects limbs and buttocks, but spares abdominal sc fat that might be increased, causing insulin resistance, diabetes, high plasma TG levels, hypertension, and in some cases liver steatosis and polycystic ovarian syndrome.
A study of the PPARγ gene in seven FPL patients revealed a heterozygous change of the highly conserved arginine 425 to cysteine, in exon 6, in one of the patients, a non-Hispanic white woman who developed type 2 diabetes and hypertriglyceridemia, and later lipodystrophy, of the extremities and face, while sc truncal fat was slightly increased 143. Since arginine 425 might be involved in a salt bridge that maintains the PPARγ protein in a proper configuration, it was speculated that this PPARγR425C mutation represents the molecular basis of one of the FPL phenotypes.
Another mutation, PPARγP467L, was found in two adult patients (man and woman) as well as a PPARγV290M mutation in a female patient. These adults also suffered from lipodystrophy at the extremities, elevated plasma TG concentrations, hyperinsulinemia, and fat accumulation in the liver. However, there was no difference in the circulating levels of leptin and TNF-α but a decrease in adiponectin levels in the two PPARγP467L patients 191. In vitro studies of both mutations suggest a destabilization of the PPARγ configuration more favorable for receptor-co-repressor interactions with dominant-negative properties. Interestingly, a PPARγ ligand stabilizes the receptor structure in the active conformation and promotes co-repressor release, which most likely explains the improvement of these patients' condition after TZD treatment 192.
In addition, four members of a same family were identified, who suffered from a transactivation deficient mutant PPARγ, namely PPARγF388L, which changes a highly conserved residue of helix 8 of the ligand-binding pocket 193. All four patients were heterozygous carriers and presented partial lipodystrophy as well as hyperinsulinemia. Moreover, the older patients suffered from type 2 diabetes and hypertension. In transactivation assays, the basal transcriptional activity of the mutant receptor was three-fold lower compared to the WT molecule in the absence of an exogenous ligand. However, in the presence of TZD, its activity increased, comparable to the WT receptor only at high rosiglitazone concentrations. It is noteworthy that the proband, when treated with pharmacological doses of rosiglitazone, in combination with metformin, had a good glycemic control 193.
Another well-studied variant is the PPARγ2P12A 70, 73, 194, 195. This is the only well-described change found so far in the N-terminal domain of PPARγ2. The initial study of Finnish and second-generation Japanese populations concluded that the less common 12A allele promotes insulin sensitivity and confers protection against type 2 diabetes 70, 73, 194, 195. In vitro studies showed that this allele reduces PPARγ DNA binding affinity and transcriptional activity 70, 73, 194, 195. Although some additional studies did not support a statistically significant role for the PPARγ2P12A polymorphism in the etiology of type 2 diabetes 196, 197, 198, a more recent meta-analysis of all published data, comprising more than 25 000 cases of diabetes, showed an association of P12A with type 2 diabetes 199. The large population that was necessary in order to demonstrate the association between P12A and type 2 diabetes is due to the weak effect of the risk allele, since individuals that are homozygous for the higher risk P12 allele have only a 25% increase in diabetes risk. However, because the frequency of the P12 allele is high in Europeans, it has a substantial effect at the level of this population, since the disease would be reduced significantly if the risk factor were not present 199. Data from a recent study support the idea that additional PPARγ variants, besides the one just described, most likely contribute to PPARγ effects on metabolic traits in African-Americans and whites 200.
In brief, the mutations found in the human PPARγ receptor show that, in general, as in mice, the level of PPARγ activity correlates with adiposity. Loss of PPARγ function is linked to partial lipodystrophy, which in turn is associated with severe metabolic dysfunctions. This connection highlights once more the role of PPARγ in the control of both lipid and glucose metabolism. Interestingly, a mild reduction in PPAR activity, as seen above with the 12A in humans, or with a partial antagonist in mice, promotes insulin sensitivity. In mice it also decreases fat depots, and brings the metabolic parameters to the levels seen in PPARγ heterozygous mice 73, 178, 180. These heterozygous mice are partially protected from high-fat diet or mono sodium glutamate-induced weight gain and insulin resistance. It is not known whether treatment of human diabetic patients with a partial PPARγ antagonist would inhibit TG accumulation in fat tissue without redistribution to muscle and liver, thus promoting insulin sensitivity 201.
PPARγ gain of function mutations
The PPARγP115Q mutation, which was found in four unrelated patients, is the only one in humans that was found to increase PPARγ activity. 115Q prevents the adjacent S114 from being phosphorylated (phosphorylation of this residue inactivates the receptor). All four patients were severely obese, lending additional support to the notion that increased PPARγ activity promotes increase in fat mass. It is noteworthy that in a nation-wide German epidemiological field survey, no individual homozygote or heterozygote for the 115Q allele was found, showing that this mutation is unlikely to have a significant epidemiological impact on morbid obesity 202. However, it certainly contributes to a better understanding of the role of PPARγ activity on fat mass in humans.
Taken together, data from the mouse models and human genetic studies underscore a direct and positive correlation between PPARγ and adiposity. Such a correlation appears less obvious between PPARγ activity and insulin sensitivity. It may suggest that insulin sensitivity is achieved mainly by a modulation of PPARγ activity within the WAT, possibly through its transcriptional effects on adipokine expression and secretion, as well as on lipogenic gene expression. Maintaining the integrity of the adipose tissue may fulfill this function. In fact, obese and lipodystrophic animals and humans both develop insulin resistance and associated pathologies.
PPARγ as a therapeutic target in fat related diseases
To become transcriptionally active, PPARγ depends on an indispensable partner, the Retinoid X Receptor, which is also a member of the nuclear hormone receptor family, with which it forms a heterodimer. This heterodimer binds to specific sequence elements in the regulatory regions of target genes, called Peroxisome Proliferator Response Elements (PPRE) and, in the presence of ligands, activates transcription. Fatty acids and prostaglandin J derivatives are natural ligands of PPARγ 4, 10.
Before even being identified formally as PPARγ ligands, TZDs were shown to stimulate adipogenesis and to improve insulin sensitivity 203, 204. It is only more recently that TZDs were described as selective ligands for the receptor, bridging the gap between PPARγ and insulin sensitivity (Figure 5) 205, 206.
Supraphysiological activation of PPARγ by TZDs stimulates adipogenesis by increasing the number of newly differentiated adipocytes, especially in the sc WAT. Increasing the storage capacity of WAT decreases ectopic lipid accumulation. The result is a decrease in liver and skeletal muscle TG content and an amelioration of insulin sensitivity at the expense of increased sc WAT mass. However, TZD amelioration of insulin sensitivity in skeletal muscle appears to be independent of the lipid profile of this organ, since the TZD treatment was shown to increase lipid accumulation in skeletal muscle 207. Lessons learned from the PPARγ+/− mouse model, as mentioned above, as well as from clinical observations of obese patients after weight loss, which correlated with reduced PPARγ levels, suggest that a moderate decrease in PPARγ activity might have beneficial effects on the TG content of WAT, liver and skeletal muscle. In fact, diminution of PPARγ transcriptional activity reduces the expression of the lipogenic program and promotes the β-oxidation pathway in the liver and muscle, with an improvement of plasma lipidic parameters as well as insulin sensitivity 179, 208, 209.
Use of synthetic PPARγ agonists in the treatment of lipodystrophies and type 2 diabetes
The effects of the TZD rosiglitazone were investigated in acquired or genetic lipodystrophies, including those caused by mutations in the PPARγ gene (see above). In patients with inherited or HIV-induced lipodystrophies, treatment with rosiglitazone increases the sc fat mass, and augments insulin sensitivity and adipokine secretion, probably by increasing the lipogenic program in WAT 175, 210, 211.
With respect to type 2 diabetes, several drugs have been introduced during the past decade, which are effective in lowering blood glucose and in reducing diabetes-related end-organ diseases. The two TZDs, rosiglitazone and pioglitazone, currently approved as antidiabetic drugs, are selective PPARγ agonists. Troglitazone, the first agent of this class, effective in controlling glycaemia, was removed from the market because of serious liver toxicity.
In addition to improving insulin signaling, rosiglitazone and pioglitazone improve cardiovascular parameters, such as lipids (increase in sc WAT, influx of FFA), blood pressure, inflammatory biomarkers (inhibition of adipokine expression and action), endothelial function, and fibrinolytic status 212, 213, 214. Furthermore, pioglitazone treatment shifts fat from the vis to the sc compartment in obese patients, a fat redistribution thought to improve insulin sensitivity since sc WAT confers less insulin resistance than vis WAT 208. These observations are consistent with recent data from a report on the treatment of male rats with a PPARγ agonist. Redistribution of fat by stimulation of the potential for lipid uptake and esterification in sc WAT was obtained, but only a minimal effect on uptake was achieved in vis WAT. More significantly, energy expenditure was strongly increased in vis fat with a consequent reduction in fat accumulation 215.
Despite the efficacy and beneficial effects of TZDs, a number of undesired side effects have been noted, including increased weight gain due to both increased adiposity and fluid retention (edema) 216, 217. The latter, which can be explained by PPARγ stimulation of ENaC-mediated renal salt absorption 218, might be the cause of an increased incidence of congestive heart failure 219, an outcome also observed in rats where rosiglitazone treatment is associated with increased post-myocardial infarction mortality 220. However, this issue is still under debate as the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) showed recently that treatment of type 2 diabetic patients with pioglitazone, another TZD drug, improved their cardiovascular outcome 113.
At the present time, substantial effort has been concentrated on generating novel selective PPARγ modulators that retain the beneficial clinical effects while avoiding the unwanted side effects.
Are PPARγ antagonists candidate drugs for type 2 diabetes?
As mentioned above, PPARγ activation reduces insulin resistance, but also increases fat mass by promoting adipocyte hypertrophy and hyperplasia. Since lasting enhanced adiposity is associated with increased insulin resistance, it was hypothesized that reducing PPARγ activity would result in less fat mass and improved insulin sensitivity. In fact, mice treated with PPARγ partial antagonists such as SR202, GW9662, or BADGE (bisphenol A diacylycidyl ether) showed decreased TG content in WAT, liver and skeletal muscle, decreased adipocyte size, increased resistance to HFD-induced obesity, and decreased expression and secretion of leptin and TNF-α. However, insulin sensitivity under HFD conditions depends on the type of antagonist that was administrated to the animals. SR202- and BADGE-treated mice presented an increased sensitivity to insulin, whereas GW9662 had no effect on insulin sensitivity 221. Especially for SR202, the effect is comparable to the improved sensitivity recorded in untreated PPARγ+/− mice, in which PPARγ levels were reduced by half compared with WT animals. 221, 222, 223, 224.
Several lines of evidence discussed above suggest that WAT dysfunctions resulting from lipodystrophies or experimentally induced loss of adiposity in animal models can also be linked to insulin resistance, possibly due to excessive levels of circulating FFA that could be lipotoxic for the liver, skeletal muscle and even for the pancreas (by affecting beta cell action). In addition, experimental disruption of the WAT in mice induces a low-grade inflammation, as in obese patients. Associated with adipokine and pro-inflammatory cytokine expression and secretion from the dysfunctional WAT, this inflammatory condition may affect insulin sensitivity. In line with this possibility, treatment of PPARγ+/− mice (where the activity of the receptor is decreased by half) with the PPARγ antagonist BADGE causes re-emergence of the lipotoxic effect of TG in liver and skeletal muscle, and insulin resistance 223.
It would appear, therefore, that the use of PPARγ antagonists over long periods of time might interfere with the integrity of the adipose tissue and trigger unexpected and undesired effects on whole-body metabolism, including insulin sensitivity.
Selective PPARγ modulators: a better alternative?
To overcome side effects associated with the use of TZDs, a novel approach consists in developing new PPARγ ligands that have insulin-sensitizing properties, via selective action on beneficial pathways, without exacerbating fluid retention and obesity. Such compounds are called “selective PPARγ-modulators” (SPPARγMs) analogous to selective estrogen receptor modulators (SERMs) such as raloxifen, which spares the uterus functions, but acts as a partial ER agonist in bones 225. SPPARγMs would separate the effects of PPARγ on lipid and glucose metabolisms as well as on different organ systems (gastrointestinal, immune, cardiovascular). The selective action of such compounds is thought to depend on different structural configurations induced in the ligand-binding domain by their interaction with the receptor, allowing recruitment of different complexes of cofactors that impact on the activation or repression of specific sets of target genes in different tissues 146, 218, 226, 227, 228, 229, 230, 231.
A variety of such new PPARγ ligands with differential pharmacological affinities for PPARγ have been reported in recent years. One of the first SPPARγMs that was tested, and which validated this idea, is FMOC-L-Leucine (F-L-Leu), which separates insulin sensitivity from adipogenesis in vivo 232.
The non-TZD-selective PPARγ modulator (nTZDpa) was also shown to alter the conformational stability of the receptor when compared to TZDs. Chronic treatment of fat-fed C57BL/6J mice with nTZDpa improved hyperglycemia and hyperinsulinemia, and promoted reductions in weight gain and adipose depot size, without causing cardiac hypertrophy. In WAT, nTZDpa produced a different in vivo expression pattern of a panel of PPAR target genes when compared to a full agonist 227. Similarly, a series of metabolically robust N-benzyl-indole partial PPARγ agonists, with either a 3-benzoyl or 3-benzisoxazoyl moiety, also produced potent glucose reduction in db/db mice and attenuated increases in heart weight and BAT mass, which are typically observed in rodents upon treatment with PPARγ full agonists 233, 234. In addition, halofenate, one of the recently discovered SPPARγMs, displays the characteristics of an optimized modulator, retaining an insulin sensitization potential with minimal adipogenic activity in vitro and with less weight gain in vivo (ob/ob mouse and fa/fa Zucker rat models). At the molecular level, the partial agonism of halofenic acid may be explained in part by effective displacement of the corepressors NCoR and SMRT, coupled with inefficient recruitment of co-activators, such as p300, CBP, and TRAP 220 235. Further characterization of SPPARγMs will most likely yield novel agents for the treatment of type 2 diabetes, which are as effective as current pharmacological compounds but without their side effects. These findings should encourage mechanism-based screens 225, which would capitalize on a still very incomplete knowledge of the ensemble of PPARγ co-activators and co-repressors and their expression profile in tissues that are pivotal for PPARγ action.
At this point, the emergence of novel potential therapeutic targets is worth mentioning, for the treatment of metabolic-related diseases, among which is the cofactor sirutin 1 (SIRT1), a protein lysine deacectylase. The human SIRT1 regulates several transcription factors that govern metabolism among which is PPARγ. SIRT1 is induced by fasting in several tissues, such as the WAT where it represses PPARγ activity thus decreasing the amount of fat storage. Its activation by resveratrol was shown to improve mitochondrial function and to protect against metabolic related diseases 236, 237, 238, 239.
Conclusion
As described herein, the adipose tissue has been promoted within only a few years from a lipid storage bag to the most sophisticated, in terms of functions, and more importantly, in terms of mass, endocrine organ of the body. It participates in the control of energy balance in two ways: firstly by managing the energy depot of the body via the timely appropriate fine-tuned uptake, storage and release of lipids, and, secondly, by communicating with many organs via an incredibly rich array of endocrine signals that are emitted or received. With this in mind, it becomes clear why the functional integrity of this organ is primordial to whole-body homeostasis as a prerequisite for good health. Treatment of metabolic disorders through modulation of PPARγ activity appears to have a promising future once knowledge has been acquired, enabling an activation/repression mechanism-based identification of PPAR isotype-selective modulators with the required characteristics. Ablation of the adipose tissue function by a selective full PPARγ antagonist is obviously not a solution. Indeed, as discussed above, animal and human models of lipodystrophy, some of which were caused by complete or partial loss of PPARγ activity, illustrate the deleterious and potentially dangerous outcome of such an approach. Supraphysiological stimulation of PPARγ activity, such as that achieved by TZDs, triggers unwanted side effects. Based on present knowledge, the demanding path of highly selective SPPARγM identification with respect to functional outcome appears to be the most promising way forward in terms of potential therapeutic benefits. Such compounds may in fact open the route to preferred therapies for type 2 diabetes, obesity, and various manifestations of the metabolic syndrome.
References
Romijn JA, Fliers E . Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Curr Opin Clin Nutr Metab Care 2005; 8:440–444.
McMillan DC, Sattar N, McArdle CS . ABC of obesity. Obesity and cancer. BMJ 2006; 333:1109–1111.
Mandrup, S, Lane MD . Regulating adipogenesis. J Biol Chem 1997; 272:5367–5370.
Juge-Aubry CE, Somm E, Giusti V, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 2003; 52:1104–1110.
Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ and Burrell MA The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 2001; 280:E827–E847.
Fried SK, Bunkin DA, Greenberg AS . Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83:847–850.
Cinti S . The adipose organ. Prostaglandins Leukot Essent Fatty Acids 2005; 73:9–15.
Hansen JB, Kristiansen K . Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J 2006; 398:153–168.
Avram AS, Avram MM, James WD . Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol 2005; 53:671–683.
Desvergne B, Wahli W . Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20:649–688.
Rosen ED, MacDougald OA . Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885–896.
Gimble JM, Zvonic S, Floyd ZE, Kassem M, and Nuttall ME . Playing with bone and fat. J Cell Biochem 2006; 98:251–266.
Crane JF, PA Trainor PA . Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol 2006; 22:267–286.
Cinti S . The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc 2001; 60:319–328.
Wajchenberg BL . Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21:697–738.
Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA 2006; 103:6676–6681.
Pouliot MC Despres JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992; 41:826–834.
Despres JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 1989; 38:304–309.
He Q, Engelson ES, DP Kotler DP . A comparison of abdominal subcutaneous adipose tissue pattern in obese and lean HIV-infected women. J Nutr 2005; 135:53–57.
Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004; 350:2549–2557.
Thorne A, Lonnqvist F, Apelman J, Hellers G, and Arner P . A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 2002; 26:193–199.
Laviola L, Perrini S, Cignarelli A, et al. Insulin signaling in human visceral and subcutaneous adipose tissue in vivo. Diabetes 2006; 55:952–961.
Park HS, Lee K . Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med 2005; 22:266–272.
Misra A, NK Vikram NK . Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003; 19:457–466.
Greenberg AS, Egan JJ, Wek SA, Moos MC Jr, Londos C, and Kimmel AR . Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci USA 1993; 90:12035–12039.
Rosen ED, Spiegelman BM . Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444:847–853.
Gibbons G . Old fat, make way for new fat. Nat Med 2005; 11:722–723.
Arner P . Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 2005; 19:471–4782.
Wang Y, Wang PY, Takashi K . Chronic effects of different non-esterified fatty acids on pancreatic islets of rats. Endocrine 2006; 29:169–173.
Joseph JW, Koshkin V, Saleh MC, et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem 2004; 279:51049–51056.
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature, 2006; 444:881–887.
Ahima RS, Qi Y, Singhal NS, Jackson MB, and Scherer PE . Brain adipocytokine action and metabolic regulation. Diabetes, 2006; 55 Suppl 2:S145–S154.
Verreth W, De Keyzer D, Pela M, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 2004; 110:3259–3269.
Ronti T, Lupattelli G, Mannarino E . The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006; 64:355–365.
Klein J, Perwitz N, Kraus D, and Fasshauer M . Adipose tissue as source and target for novel therapies. Trends Endocrinol Metab 2006; 17:26–32.
Juge-Aubry CE, Henrichot E, Meier CA . Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab 2005; 19:547–566.
Hutley L, Prins JB . Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci 2005; 330:280–289.
Guerre-Millo M . Adipose tissue and adipokines: for better or worse. Diabetes Metab 2004; 30:13–19.
Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269:543–546.
Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, and Flier JS . Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1995; 1:1311–1314.
Unger RH . Hyperleptinemia: protecting the heart from lipid overload. Hypertension 2005; 45:1031–1034.
Wang MY, Orci L, Ravazzola M, and Unger RH . Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA 2005; 102:18011–18016.
Kershaw EE, JS Flier JS . Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89:2548–2556.
Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007; 92:532–541.
Pelleymounter MA, Cullen MJ, Baker MB . Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995; 269:540–543.
Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999; 282:1568–1575.
Flores-Morales A, Greenhalgh CJ, Norstedt G, and Rico-Bautista E . Negative regulation of growth hormone receptor signaling. Mol Endocrinol, 2006; 20:241–253.
Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002; 110:1093–1103.
Akirav EM, Chan O, Inouye K, Riddell MC, Matthews SG, and Vranic M . Partial leptin restoration increases hypothalamic-pituitary-adrenal activity while diminishing weight loss and hyperphagia in streptozotocin diabetic rats. Metabolism 2004; 53:1558–1564.
Lloyd RV, Jin L, Tsumanuma I, et al. Leptin and leptin receptor in anterior pituitary function. Pituitary 2001; 4:33–47.
Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006; 281:26602–26614.
Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, and Lodish HF . T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 2004; 101:10308–10313.
Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, and Libby P . Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006; 110:267–278.
Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941–946.
Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8:1288–1295.
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001; 50:1126–1133.
Yatagai T, Nagasaka S, Taniguchi A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism 2003; 52:1274–1278.
Steppan CM, Brown EJ, Wright CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 2001; 98:502–506.
Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science 2004; 303:1195–1198.
Patel SD, Rajala MW, Rossetti L, Scherer PE, and Shapiro L . Disulfide-dependent multimeric assembly of resistin family hormones. Science 2004; 304:1154–1158.
Kusminski CM, McTernan PG, Kumar S . Role of resistin in obesity, insulin resistance and type II diabetes. Clin Sci (Lond) 2005; 109:243–256.
Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001; 409:307–312.
Fain JN, Cheema PS, Bahouth SW, and Lloyd Hiler M . Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun 2003; 300:674–678.
Nagaev I, Smith U . Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 2001; 285:561–564.
Savage DB, Sewter CP, Klenk ES, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes 2001; 50:2199–2202.
Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003; 300:472–476.
Kielstein JT, Becker B, Graf S, Brabant G, Haller H, and Fliser D . Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis 2003; 42:62–66.
Banerjee RR, MA Lazar MA . Resistin: molecular history and prognosis. J Mol Med 2003; 81:218–226.
Kersten S . Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans 2005; 33 Part 5:1059–1062.
Kim I, Kim HG, Kim H, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J 2000; 346 Part 3:603–610.
Puigserver P, Rhee J, Lin J, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 2001; 8:971–982.
Kersten S, Mandard S, Tan NS, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 2000; 275:28488–28493.
Hara K, Okada T, Tobe K, et al. The Pro12Ala polymorphism in PPAR gamma2 may confer resistance to type 2 diabetes. Biochem Biophys Res Commun 2000; 271:212–216.
Xu A, Lam MC, Chan KW, et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA 2005; 102:6086–6091.
Mandard S, Zandbergen F, van Straten E, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem 2006; 281:934–944.
Lee K, Villena JA, Moon YS, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest 2003; 111:453–461.
Greenberg AS, MS Obin MS . Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83:461S–465S.
Yu YH, Ginsberg HN . Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res 2005; 96:1042–1052.
Yang RZ, Lee MJ, Hu H, Pollin TI, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 2006; 3:e287.
Kahn SE, Zinman B, Haffner SM, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 2006; 55:2357–2364.
Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 2005; 11:797–803.
Sadler D, Mattacks CA, CM Pond CM . Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J Anat 2005; 207:769–781.
Wellen KE, GS Hotamisligil GS . Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003; 112:1785–1788.
Hotamisligil GS, Shargill NS, BM Spiegelman BM . Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:87–91.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444:860–867.
Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116:115–124.
Winkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol 2003; 149:129–135.
Bullo M, Garcia-Lorda P, Peinado-Onsurbe J, et al. TNFalpha expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord 2002; 26:652–658.
Prins JB, Niesler CU, Winterford CM, et al. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes 1997; 46:1939–1944.
Ruan H, Miles PD, Ladd CM, et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 2002; 51:3176–3188.
Cheung AT, Ree D, Kolls JK, Fuselier J, Coy DH, and Bryer-Ash M . An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology 1998; 139:4928–4935.
Van der Poll T, Romijn JA, Endert E, Borm JJ, Buller HR, and Sauerwein HP . Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol 1991; 261(4 Part 1):E457–E465.
Zhang YH, Lin JX, Vilcek J . Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol 1990; 10:3818–3823.
Bastard JP, Maachi M, Van Nhieu JT, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 2002; 87:2084–2089.
Keller P, Penkowa M, Keller C, et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J 2005; 19:1181–1183.
Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005; 111:996–1005.
Klover PJ, Zimmers TA, Koniaris LG, and Mooney RA . Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 2003; 52:2784–2789.
Scaglione R, Argano C, di Chiara T, et al. Central obesity and hypertensive renal disease: association between higher levels of BMI, circulating transforming growth factor beta1 and urinary albumin excretion. Blood Press 2003; 12:269–276.
Fain JN, Tichansky DS, Madan AK . Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005; 54:1546–1551.
Alessi MC, Bastelica D, Morange P, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000; 49:1374–1380.
Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54:2277–2286.
Christiansen T, Richelsen B, Bruun JM . Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005; 29:146–150.
Sell H, Dietze-Schroeder D, Kaise U, and Eckel J . Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 2006; 147:2458–2467.
Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116:1494–1505.
Pandey M, Loskutoff DJ, Samad F . Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J 2005; 19:1317–1319.
Juhan-Vague I, Alessi MC, Mavri A, and Morange PE . Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1:1575–1579.
Alessi MC, Juhan-Vague I . PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol 2006; 26:2200–2207.
Loskutoff DJ, Samad F . The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol 1998; 18:1–6.
Lundgren CH, Brown SL, Nordt TK, Sobel BE, and Fujii S . Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 1996; 93:106–110.
Skurk T, Hauner H . Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord 2004; 28:1357–1364.
Samad F, Pandey M, Loskutoff DJ . Tissue factor gene expression in the adipose tissues of obese mice. Proc Natl Acad Sci USA 1998; 95:7591–7596.
Zhang Y, Deng Y, Luther T, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest 1994; 94:1320–1327.
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289.
Prasad A, Quyyumi AA . Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation 2004; 110:1507–1512.
Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, and Wise PM . Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 2006; 27:575–605.
Farnier C, Krief S, Blache M, et al. Adipocyte functions are modulated by cell size change: potential involvement of an integrin/ERK signalling pathway. Int J Obes Relat Metab Disord 2003; 27:1178–1186.
DiGirolamo M, Fine JB, Tagra K, and Rossmanith R . Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol 1998; 274(5 Part 2):R1460–R1467.
Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Networks 2006; 17:4–12.
Suganami T, Nishida J, Ogawa Y . A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005; 25:2062–2068.
Neels JG, Olefsky JM . Inflamed fat: what starts the fire? J Clin Invest 2006; 116:33–35.
Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112:1821–1830.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112(12):1796–1808.
Clement K, Viguerie N, Poitou C, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004; 18:1657–1669.
Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46:2347–2355.
Sartipy P, Loskutoff DJ . Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 2003; 100:7265–7270.
Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, and Pratley RE . Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 2001; 9:414–417.
Clement K . Genetics of human obesity. C R Biol 2006; 329:608–622; discussion 653–655.
Simha V, Garg A . Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol 2006; 17: 162–169.
Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, and Weiss SJ . A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 2006; 125: 577–5791.
Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 2000; 24:153–156.
Morel CF, Thomas MA, Cao H, et al. A LMNA splicing mutation in two sisters with severe Dunnigan-type familial partial lipodystrophy type 2. J Clin Endocrinol Metab 2006; 91:2689–2695.
Hegele RA, Kraw ME, Ban MR, Miskie BA, Huff MW, and Cao H . Elevated serum C-reactive protein and free fatty acids among nondiabetic carriers of missense mutations in the gene encoding lamin A/C (LMNA) with partial lipodystrophy. Arterioscler Thromb Vasc Biol 2003; 23:111–116.
Hegele RA, Anderson CM, Wang J, Jones DC, and Cao H . Association between nuclear lamin A/C R482Q mutation and partial lipodystrophy with hyperinsulinemia, dyslipidemia, hypertension, and diabetes. Genome Res 2000; 10:652–658.
Wong SP, Huda M, English P, et al. Adipokines and the insulin resistance syndrome in familial partial lipodystrophy caused by a mutation in lamin A/C. Diabetologia 2005; 48:2641–2649.
Lundin C, Nordstrom R, Wagner K, et al. Membrane topology of the human seipin protein. FEBS Lett 2006; 580:2281–2284.
Magre J, Delepine M, Van Maldergem L, et al. Prevalence of mutations in AGPAT2 among human lipodystrophies. Diabetes 2003; 52:1573–1578.
Fischer-Posovszky P, Hebestreit H, Hofmann AK, et al. Role of CD95-mediated adipocyte loss in autoimmune lipodystrophy. J Clin Endocrinol Metab 2006; 91:1129–1135.
Domingo P, Vidal F, Domingo JC, et al. Tumour necrosis factor alpha in fat redistribution syndromes associated with combination antiretroviral therapy in HIV-1-infected patients: potential role in subcutaneous adipocyte apoptosis. Eur J Clin Invest 2005; 35:771–780.
Jan V, Cervera P, Maachi M, et al. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther 2004; 9:555–564.
Domingo P, Matias-Guiu X, Pujol RM, et al. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. Aids 1999; 13:2261–2267.
Imai T, Takakuwa R, Marchand S, et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA 2004; 101:4543–4547.
Yki-Jarvinen H . Thiazolidinediones. N Engl J Med 2004; 351:1106–1118.
Agarwal AK, Garg A . A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 2002; 87:408–411.
Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, and Wahli W . Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 1992; 68:879–887.
Issemann I, Green S . Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990; 347:645–650.
Feige JN, Gelman L, Michalik L, Desvergne B, and Wahli W . From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 2006; 45: 120–159.
Marques BG, Hausman DB, Martin RJ . Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am J Physiol 1998; 275(6 Part 2):R1898–R1908.
Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, and Zamora S . Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006; 30:899–905.
Crossno JT, Majka SM, Grazia T, Gill RG, and Klemm DJ . Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest 2006; 116:3220–3228.
Hausman GJ, Hausman DB . Search for the preadipocyte progenitor cell. J Clin Invest 2006; 116:3103–3106.
Chawla A, Schwarz EJ, Dimaculangan DD, and Lazar MA . Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994; 135:798–800.
Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, and Desvergne B . Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 2001; 142:4195–4202.
Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, and Dube G P . Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 2001; 224:29–37.
Tontonoz P, Hu E, Graves RA, Budavari AI, and Spiegelman BM . mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 1994; 8:1224–1234.
Lehrke M, Lazar MA . The many faces of PPARgamma. Cell 2005; 123:993–999.
Gerhold DL, Liu F, Jiang G, et al. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrinology 2002; 143:2106–2118.
Ross SE, Erickson RL, Gerin I, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor alpha in adipocyte metabolism. Mol Cell Biol 2002; 22:5989–5999.
Patel YM, Lane MD . Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J Biol Chem 2000; 275:17653–17660.
Hosono T, Mizuguchi H, Katayama K, et al. RNA interference of PPARgamma using fiber-modified adenovirus vector efficiently suppresses preadipocyte-to-adipocyte differentiation in 3T3-L1 cells. Gene 2005; 348:157–165.
Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999; 4:611–617.
Tontonoz P, Hu E, Spiegelman BM . Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79:1147–1156.
Farmer SR . Transcriptional control of adipocyte formation. Cell Metab 2006; 4:263–273.
Way JM, Harrington WW, Brown KK, et al. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor gamma activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology 2001; 142:1269–1277.
Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 1996; 15:5336–5348.
Avallone R, Demers A, Rodrigue-Way A, et al. A growth hormone-releasing peptide that binds scavenger receptor CD36 and ghrelin receptor upregulates ABC sterol transporters and cholesterol efflux in macrophages through a PPAR{gamma}-dependent pathway. Mol Endocrinol 2006; 20:3165–78.
Martin G, Poirier H, Hennuyer N, et al. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem 2000; 275:12612–12618.
Bogacka I, Xie H, Bray GA, and Smith SR . The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care 2004; 27:1660–1667.
Schachtrup C Emmler T, Bleck B, Sandqvist A, and Spener F . Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochem J 2004; 382(Part 1):239–245.
Ranganathan G, Unal R, Pokrovskaya I, et al. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res 2006; 47:2444–2450.
Dalen KT, Schoonjans K, Ulven SM, et al. Adipose tissue expression of the lipid droplet-associating proteins S3–12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes 2004; 53:1243–1252.
Patsouris D, Mandard S, Voshol PJ, et al. PPARalpha governs glycerol metabolism. J Clin Invest 2004; 114:94–103.
Olswang Y, Cohen H, Papo O, et al. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci USA 2002; 99:625–630.
Guan HP, Li Y, Jensen MV, Newgard CB, Steppan CM, and Lazar MA . A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med 2002; 8:1122–1128.
Tontonoz P, Hu E, Devine J, Beale EG, and Spiegelman BM . PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 1995; 15:351–357.
Riserus U, Tan GD, Fielding BA et al. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes 2005; 54:1379–1384.
Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4:585–595.
Duan SZ, Ivashchenko CY, Whitesall SE, et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J Clin Invest 2007; 117:812–822.
Rieusset J, Seydoux J, Anghel SI, et al. Altered growth in male peroxisome proliferator-activated receptor gamma (PPARgamma) heterozygous mice: involvement of PPARgamma in a negative feedback regulation of growth hormone action. Mol Endocrinol, 2004; 18:2363–2377.
Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 2001; 276:41245–41254.
Miles PD, Barak Y, He W, Evans RM, and Olefsky JM . Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest 2000; 105:287–292.
Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 1999; 4:597–609.
He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 2003; 100:15712–15717.
Freedman BD, Lee EJ, Park Y, and Jameson JL . A dominant negative peroxisome proliferator-activated receptor-gamma knock-in mouse exhibits features of the metabolic syndrome. J Biol Chem 2005; 280:17118–17125.
Jones JR, Barrick C, Kim KA, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 2005; 102:6207–6212.
Zhang J, Fu M, Cui T, et al. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA 2004; 101:10703–10708.
Koutnikova H, Cock TA, Watanabe M, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA 2003; 100:14457–14462.
Chao L, Marcus-Samuels B, Mason MM, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest 2000; 106:1221–1228.
Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 2003; 278:34268–34276.
Kim JK, Fillmore JJ, Gavrilova O, et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes 2003; 52:1311–1318.
Ailhaud G . Adipose tissue as a secretory organ: from adipogenesis to the metabolic syndrome. C R Biol 2006; 329: 570–577; discussion 653–655.
Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 2003; 52:910–917.
Agostini M, Gurnell M, Savage DB, et al. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor gamma. Endocrinology 2004; 145:1527–1538.
Hegele RA, Cao H, Frankowski C, Mathews ST, and Leff T . PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes 2002; 51:3586–3590.
Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000; 26:76–80.
Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998; 20:284–287.
Meirhaeghe A, Fajas L, Helbecque N, et al. Impact of the peroxisome proliferator activated receptor gamma2 Pro12Ala polymorphism on adiposity, lipids and non-insulin-dependent diabetes mellitus. Int J Obes Relat Metab Disord 2000; 24:195–199.
Clement K, Hercberg S, Passinge B, et al. The Pro115Gln and Pro12Ala PPAR gamma gene mutations in obesity and type 2 diabetes. Int J Obes Relat Metab Disord 2000; 24:391–393.
Mancini FP, Vaccaro O, Sabatino L, et al. Pro12Ala substitution in the peroxisome proliferator-activated receptor-gamma2 is not associated with type 2 diabetes. Diabetes 1999; 48:1466–1468.
Florez JC, Hirschhorn J, Altshuler D . The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet 2003; 4:257–291.
Weedon MN, McCarthy MI, Hitman G, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med 2006; 3:e374.
Desvergne B, Michalik L, Wahli W . Be fit or be sick: peroxisome proliferator-activated receptors are down the road. Mol Endocrinol 2004; 18:1321–1332.
Hamer OW, Forstner D, Ottinger I, et al. The Pro115Gln polymorphism within the PPAR gamma2 gene has no epidemiological impact on morbid obesity. Exp Clin Endocrinol Diabetes 2002; 110:230–234.
Hiragun A, Sato M, Mitsui H . Preadipocyte differentiation in vitro: identification of a highly active adipogenic agent. J Cell Physiol 1988; 134:124–130.
Fujita T, Sugiyama Y, Taketomi S, et al. Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, ciglitazone), a new antidiabetic agent. Diabetes 1983; 32:804–810.
Lazar MA . PPAR gamma, 10 years later. Biochimie 2005; 87:9–13.
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM and Kliewer SA . An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995; 270:12953–12956.
Todd MK, Watt MJ, Le J, Hevener AL, and Turcotte LP . Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 2007; 292:E485–E493.
Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002; 87:2784–2791.
Bastard JP, Hainque B, Dusserre E, et al. Peroxisome proliferator activated receptor-gamma, leptin and tumor necrosis factor-alpha mRNA expression during very low calorie diet in subcutaneous adipose tissue in obese women. Diabetes Metab Res Rev 1999; 15:92–98.
Ludtke A, Heck K, Genschel J, et al. Long-term treatment experience in a subject with Dunnigan-type familial partial lipodystrophy: efficacy of rosiglitazone. Diabet Med 2005; 22:1611–1613.
van Wijk JP, de Koning EJ, Cabezas MC, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med 2005; 143:337–346.
Sarafidis PA, Lasaridis AN . Actions of peroxisome proliferator-activated receptors-gamma agonists explaining a possible blood pressure-lowering effect. Am J Hypertens 2006; 19:646–653.
Hetzel J, Balletshofer B, Rittig K, et al. Rapid effects of rosiglitazone treatment on endothelial function and inflammatory biomarkers. Arterioscler Thromb Vasc Biol 2005; 25:1804–1809.
Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, and Cheung P . Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes 2005; 54:880–885.
Laplante M, Festuccia WT, Soucy G, et al. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes 2006; 55:2771–2778.
Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003; 108:2941–2948.
Lebovitz HE, Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev 2002; 18 Suppl 2:S23–S29.
Guan Y, Hao C, Cha DR, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med 2005; 11:861–866.
Delea TE, Edelsberg JS, Hagiwara M, Oster G, and Phillips LS . Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care 2003; 26:2983–2989.
Lygate CA, Hulbert K, Monfared M, et al. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc Res 2003; 58:632–637.
Nakano R, Kurosaki E, Yoshida S, et al. Antagonism of peroxisome proliferator-activated receptor gamma prevents high-fat diet-induced obesity in vivo. Biochem Pharmacol 2006; 72:42–52.
Rieusset J, Touri F, Michalik L, et al. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol 2002; 16:2628–2644.
Yamauchi T, Waki H, Kamon J, et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest 2001; 108:1001–1013.
Wright HM, Clish CB, Mikami T, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem 2000; 275:1873–1877.
McDonnell DP . Mechanism-based discovery as an approach to identify the next generation of estrogen receptor modulators. FASEB J 2006; 20:2432–2434.
Schupp M, Clemenz M, Gineste R, et al. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes 2005; 54:3442–3452.
Berger JP, Petro AE, Macnaul KL, et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein [gamma] selective modulator. Mol Endocrinol 2003; 17:662–676.
Gurnell M, Savage DB, Chatterjee VK, and O'Rahilly S . The metabolic syndrome: peroxisome proliferator-activated receptor gamma and its therapeutic modulation. J Clin Endocrinol Metab 2003; 88:2412–2421.
Wang M, Tafuri S . Modulation of PPARgamma activity with pharmaceutical agents: treatment of insulin resistance and atherosclerosis. J Cell Biochem 2003; 89:38–47.
Sporn MB, Suh N, Mangelsdorf DJ . Prospects for prevention and treatment of cancer with selective PPARgamma modulators (SPARMs). Trends Mol Med 2001; 7:395–400.
Jordan VC, Morrow M . Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr Rev 1999; 20:253–278.
Rocchi S, Picard F, Vamecq J, et al. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell 2001; 8:737–747.
Liu K, Black RM, Acton JJ, et al. Selective PPARgamma modulators with improved pharmacological profiles. Bioorg Med Chem Lett 2005; 15:2437–2440.
Acton JJ III, Black RM, Jones AB, et al. Benzoyl 2-methyl indoles as selective PPARgamma modulators. Bioorg Med Chem Lett 2005; 15:357–362.
Allen T, Zhang F, Moodie SA, et al. Halofenate is a selective peroxisome proliferator-activated receptor gamma modulator with antidiabetic activity. Diabetes 2006; 55:2523–2533.
Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004; 429:771–776.
Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444:337–342.
Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127:1109–1122.
Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004; 305:390–392.
Tortora GJ, Grawsik SR, eds. Principes d'anatomie et de physiologie. 3rd Edition. Bruxelles: DeBoek Université, 2001:1252.
Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005; 307:426–430.
Van Harmelen V, Reynisdottir S, Cianflone K, et al. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem 1999; 274:18243–18251.
Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436:356–362.
Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA 2005; 102:10610–10615.
Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 2006; 290:E1253–E1261.
Sorhede Winzell M, Magnusson C, Ahren B . The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept 2005; 131:12–17.
Fain JN, Madan AK, Hiler ML, Cheema P, and Bahouth SW . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004; 145:2273–82.
Somm E, Cettour-Rose P, Asensio C, et al. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia 2006; 49:387–393.
Straczkowski M, Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, and Kinalska I . Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab 2002; 87:4602–4606.
Herder C, Haastert B, Muller-Scholze S, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Diabetes 2005; 54 Suppl 2:S11–S17.
Herder C, Hauner H, Kempf K, Kolb H, and Skurk T . Constitutive and regulated expression and secretion of interferon-gamma-inducible protein 10 (IP-10/CXCL10) in human adipocytes. Int J Obes (Lond) 2006; 31:403–410.
Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, and Carlsson LM . Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 1998; 83:3925–3929.
Massiera F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 2001; 15:2727–2729.
Berg AH, PE Scherer . Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005; 96:939–949.
Acknowledgements
We are very grateful to Vittorio Giusti (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) and Liliane Michalik (Center for Integrative Genomics, Lausanne, Switzerland) for critical reading of the manuscript and to Nathalie Constantin (Center for Integrative Genomics, Lausanne, Switzerland) for help in manuscript preparation. The work done in the authors' laboratory was supported by the Etat de Vaud and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anghel, S., Wahli, W. Fat poetry: a kingdom for PPARγ. Cell Res 17, 486–511 (2007). https://doi.org/10.1038/cr.2007.48
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2007.48
Keywords
This article is cited by
-
The spectrum of T cell metabolism in health and disease
Nature Reviews Immunology (2018)
-
Commensal gut bacteria modulate phosphorylation-dependent PPARγ transcriptional activity in human intestinal epithelial cells
Scientific Reports (2017)
-
Glucose intolerance after chronic stress is related with downregulated PPAR-γ in adipose tissue
Cardiovascular Diabetology (2016)
-
Crystal structure of FabZ-ACP complex reveals a dynamic seesaw-like catalytic mechanism of dehydratase in fatty acid biosynthesis
Cell Research (2016)
-
Intestinal PPARγ signalling is required for sympathetic nervous system activation in response to caloric restriction
Scientific Reports (2016)