Abstract
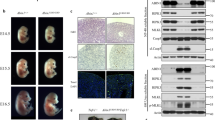
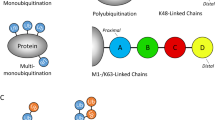
Proteins that directly regulate tumour necrosis factor receptor (TNFR) signalling have critical roles in regulating cellular activation and survival. ABIN-1 (A20 binding and inhibitor of NF-κB) is a novel protein that is thought to inhibit NF-κB signalling1,2. Here we show that mice deficient for ABIN-1 die during embryogenesis with fetal liver apoptosis, anaemia and hypoplasia. ABIN-1 deficient cells are hypersensitive to tumour necrosis factor (TNF)-induced programmed cell death, and TNF deficiency rescues ABIN-1 deficient embryos. ABIN-1 inhibits caspase 8 recruitment to FADD (Fas-associated death domain-containing protein) in TNF-induced signalling complexes, preventing caspase 8 cleavage and programmed cell death. Moreover, ABIN-1 directly binds polyubiquitin chains and this ubiquitin sensing activity is required for ABIN-1’s anti-apoptotic activity. These studies provide insights into how ubiquitination and ubiquitin sensing proteins regulate cellular and organismal survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Heyninck, K. et al. The zinc finger protein A20 inhibits TNF-induced NF-κB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-κB-inhibiting protein ABIN-1. J. Cell Biol. 145, 1471–1482 (1999)
Opipari, A. W., Boguski, M. S. & Dixit, V. M. The A20 cDNA induced by tumor necrosis factor α encodes a novel type of zinc finger protein. J. Biol. Chem. 265, 14705–14708 (1990)
Doi, T. S. et al. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl Acad. Sci. USA 96, 2994–2999 (1999)
Beg, A. A. & Baltimore, D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 (1996)
Wang, C. Y., Mayo, M. W. & Baldwin, A. S. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 274, 784–787 (1996)
Van Antwerp, D. J., Martin, S. J., Kafri, T., Green, D. R. & Verma, I. M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274, 787–789 (1996)
Wullaert, A. et al. Adenoviral gene transfer of ABIN-1 protects mice from TNF/galactosamine-induced acute liver failure and lethality. Hepatology 42, 381–389 (2005)
Barnhart, B. C., Alappat, E. C. & Peter, M. E. The CD95 type I/type II model. Semin. Immunol. 15, 185–193 (2003)
De Smaele, E. et al. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001)
Tang, G. et al. Inhibition of JNK activation through NF-κB target genes. Nature 414, 313–317 (2001)
Chang, L. et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124, 601–613 (2006)
Lee, E. G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000)
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003)
Schneider-Brachert, W. et al. Compartmentalization of TNF receptor 1 signaling: Internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 (2004)
Heyninck, K., Kreike, M. M. & Beyaert, R. Structure-function analysis of the A20-binding inhibitor of NF-κ B activation, ABIN-1. FEBS Lett. 536, 135–140 (2003)
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006)
Wu, C. J., Conze, D. B., Li, T., Srinivasula, S. M. & Ashwell, J. D. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation. Nature Cell Biol. 8, 398–406 (2006)
Wagner, S. et al. Ubiquitin binding mediates the NF-κB inhibitory potential of ABIN proteins. Oncogene 27, 3739–3745 (2008)
Deng, L. et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000)
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001)
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biol. 7, 758–765 (2005)
Wertz, I. E. & Dixit, V. M. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 19, 313–324 (2008)
Lee, J. C. & Peter, M. E. Regulation of apoptosis by ubiquitination. Immunol. Rev. 193, 39–47 (2003)
Boone, D. L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nature Immunol. 5, 1052–1060 (2004)
Acknowledgements
This work was supported by the NIH (A.M.), UCSF Liver Center Pathology and Flow Cytometry Facilities, UCSF Transgenic and Targeted Mutagenesis Core Facility, a fellowship from the Crohn’s and Colitis Foundation of America (S.O.), a pre-doctoral NSF fellowship (J.A.C.) and the Rainin Foundation.
Author Contributions S.O. and E.E.T. contributed equally to this work. S.O. and J.A.C. performed the cell signalling experiments. J.A.C., E.E.T. and S.O. analysed Tnip1-/- embryos. S.O. performed DISC analyses. E.E.T. performed the ubiquitin binding experiments. E.E.T. and S.C. designed and generated the ABIN-1 gene targeting construct. B.A.M. generated Tnip1-/- ES cells and mice with assistance from S.C. R.A. genotyped and maintained Tnip1-/- mice and performed timed matings. J.B., N.S., B.L. and T.W. performed mutagenesis and generated expression constructs. B.Y. analysed histological and immunohistochemical studies of Tnip1-/- embryos. A.M. and B.A.M. supervised the overall project, and wrote and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3 with Legends. (PDF 363 kb)
Rights and permissions
About this article
Cite this article
Oshima, S., Turer, E., Callahan, J. et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature 457, 906–909 (2009). https://doi.org/10.1038/nature07575
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07575
This article is cited by
-
Ubiquitin-binding domain in ABIN1 is critical for regulating cell death and inflammation during development
Cell Death & Differentiation (2022)
-
A20 and ABIN-1 cooperate in balancing CBM complex-triggered NF-κB signaling in activated T cells
Cellular and Molecular Life Sciences (2022)
-
Cell survival and DNA damage repair are promoted in the human blood thanatotranscriptome shortly after death
Scientific Reports (2021)
-
ABIN-1 is a key regulator in RIPK1-dependent apoptosis (RDA) and necroptosis, and ABIN-1 deficiency potentiates necroptosis-based cancer therapy in colorectal cancer
Cell Death & Disease (2021)
-
Met1-linked ubiquitin signalling in health and disease: inflammation, immunity, cancer, and beyond
Cell Death & Differentiation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.