Abstract
G-protein-coupled receptors (GPCRs) are critically regulated by β-arrestins, which not only desensitize G-protein signalling but also initiate a G-protein-independent wave of signalling1,2,3,4,5. A recent surge of structural data on a number of GPCRs, including the β2 adrenergic receptor (β2AR)–G-protein complex, has provided novel insights into the structural basis of receptor activation6,7,8,9,10,11. However, complementary information has been lacking on the recruitment of β-arrestins to activated GPCRs, primarily owing to challenges in obtaining stable receptor–β-arrestin complexes for structural studies. Here we devised a strategy for forming and purifying a functional human β2AR–β-arrestin-1 complex that allowed us to visualize its architecture by single-particle negative-stain electron microscopy and to characterize the interactions between β2AR and β-arrestin 1 using hydrogen–deuterium exchange mass spectrometry (HDX-MS) and chemical crosslinking. Electron microscopy two-dimensional averages and three-dimensional reconstructions reveal bimodal binding of β-arrestin 1 to the β2AR, involving two separate sets of interactions, one with the phosphorylated carboxy terminus of the receptor and the other with its seven-transmembrane core. Areas of reduced HDX together with identification of crosslinked residues suggest engagement of the finger loop of β-arrestin 1 with the seven-transmembrane core of the receptor. In contrast, focal areas of raised HDX levels indicate regions of increased dynamics in both the N and C domains of β-arrestin 1 when coupled to the β2AR. A molecular model of the β2AR–β-arrestin signalling complex was made by docking activated β-arrestin 1 and β2AR crystal structures into the electron microscopy map densities with constraints provided by HDX-MS and crosslinking, allowing us to obtain valuable insights into the overall architecture of a receptor–arrestin complex. The dynamic and structural information presented here provides a framework for better understanding the basis of GPCR regulation by arrestins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pierce, K. L. & Lefkowitz, R. J. Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nature Rev. Neurosci. 2, 727–733 (2001)
Shukla, A. K., Xiao, K. & Lefkowitz, R. J. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 36, 457–469 (2011)
Lefkowitz, R. J. & Shenoy, S. K. Transduction of receptor signals by β-arrestins. Science 308, 512–517 (2005)
Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nature Rev. Mol. Cell Biol. 3, 639–650 (2002)
DeWire, S. M., Ahn, S., Lefkowitz, R. J. & Shenoy, S. K. β-Arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 (2007)
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011)
Weis, W. I. & Kobilka, B. K. Structural insights into G-protein-coupled receptor activation. Curr. Opin. Struct. Biol. 18, 734–740 (2008)
Rosenbaum, D. M., Rasmussen, S. G. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009)
Westfield, G. H. et al. Structural flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. Proc. Natl Acad. Sci. USA 108, 16086–16091 (2011)
Rasmussen, S. G. et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450, 383–387 (2007)
Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 (2011)
Oakley, R. H., Laporte, S. A., Holt, J. A., Caron, M. G. & Barak, L. S. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 275, 17201–17210 (2000)
Shukla, A. K. et al. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141 (2013)
Goodman, O. B., Jr et al. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383, 447–450 (1996)
Nobles, K. N., Guan, Z., Xiao, K., Oas, T. G. & Lefkowitz, R. J. The active conformation of β-arrestin1: direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of β-arrestins1 and -2. J. Biol. Chem. 282, 21370–21381 (2007)
Xiao, K., Shenoy, S. K., Nobles, K. & Lefkowitz, R. J. Activation-dependent conformational changes in β-arrestin 2. J. Biol. Chem. 279, 55744–55753 (2004)
Gurevich, V. V. & Gurevich, E. V. The molecular acrobatics of arrestin activation. Trends Pharmacol. Sci. 25, 105–111 (2004)
Chung, K. Y. et al. Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature 477, 611–615 (2011)
Konermann, L., Pan, J. & Liu, Y. H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem. Soc. Rev. 40, 1224–1234 (2011)
Kim, M. et al. Conformation of receptor-bound visual arrestin. Proc. Natl Acad. Sci. USA 109, 18407–18412 (2012)
Hanson, S. M. et al. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc. Natl Acad. Sci. USA 103, 4900–4905 (2006)
Zhuang, T. et al. Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc. Natl Acad. Sci. USA 110, 942–947 (2013)
Gimenez, L. E., Vishnivetskiy, S. A., Baameur, F. & Gurevich, V. V. Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J. Biol. Chem. 287, 29495–29505 (2012)
Gurevich, V. V. & Gurevich, E. V. Structural determinants of arrestin functions. Prog. Mol. Biol. Transl. Sci. 118, 57–92 (2013)
Lohse, M. J. & Hoffmann, C. Arrestin interactions with G protein-coupled receptors. Handb. Exp. Pharmacol. 219, 15–56 (2014)
Radermacher, M., Wagenknecht, T., Verschoor, A. & Frank, J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J. Microsc. 146, 113–136 (1987)
Kim, K. M. & Caron, M. G. Complementary roles of the DRY motif and C-terminus tail of GPCRS for G protein coupling and β-arrestin interaction. Biochem. Biophys. Res. Commun. 366, 42–47 (2008)
Yang, Z., Fang, J., Chittuluru, J., Asturias, F. J. & Penczek, P. A. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure 20, 237–247 (2012)
Kang, D. S. et al. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J. Biol. Chem. 284, 29860–29872 (2009)
Haas, W. et al. Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol. Cell. Proteomics 5, 1326–1337 (2006)
Yang, B. et al. Identification of cross-linked peptides from complex samples. Nature Methods 9, 904–906 (2012)
Ohi, M., Li, Y., Cheng, Y. & Walz, T. Negative staining and image classification—powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004)
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999)
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996)
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003)
Grigorieff, N. FREALIGN: high-resolution refinement of single particle structures. J. Struct. Biol. 157, 117–125 (2007)
Acknowledgements
We thank D. Capel for technical assistance, V. Ronk, D. Addison and Q. Lennon for administrative support, R. K. Sunahara for stimulating discussions and Alex R. B. Thomsen for critical reading of the manuscript. We acknowledge support from the National Institutes of Health Grants DK090165 (G.S.), NS028471 (B.K.K.), GM072688 and GM087519 (A.A.K. and S.K.), HL075443 (K.X.), HL16037 and HL70631 (R.J.L.), from the Mathers Foundation (B.K.K.), GM60635 (P.A.P.) and from the Pew Scholars Program in Biomedical Sciences (G.S.). R.H. and S.S.S. were supported by a research grant from the Canadian Institutes of Health Research (MOP-93725). R.I.R is supported by a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. R.J.L. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.K.S. designed and optimized procedures for forming and purifying the complex, executed and optimized the on-column crosslinking protocol and provided the preparations of complex used for EM, HDX-MS and crosslink mapping experiments with assistance from P.T.-S. R.I.R. and L.-Y.H. performed biochemical and pharmacological characterization of the complex. G.H.W. performed EM analysis assisted by M.S., A.N.O. and A.M.D. and supervised by G.S. K.X. performed the HDX-MS experiments assisted by S.L., J.Q., A.W.K. and A.B., performed the crosslink mapping experiments assisted by J.Q. and A.W.K., and designed the disulphide trapping experiments carried out by M.C. V.L.W. Jr supervised the initial phase of the HDX-MS experiments. C.-R.L., L.-L.G., J.-M.S. and X.C. synthesized the high-affinity agonist BI-167107. R.H. and S.S.S. provided the linker sequence, vector and advice on ScFv conversion and expression. X.J.Y. and B.U.K. contributed in assessing various methods of complex formation. P.A.P. provided advice on implementation of ISAC28. S.K. and A.A.K. provided the phage display library and protocols for Fab selection, expression and purification. B.K.K. conceived the on-column crosslinking strategy, advised A.K.S. on its execution and optimization, assisted with molecular modelling of the complex and participated in supervision of the project. G.S. directly supervised the EM studies, performed the molecular modelling of the complex and supervised overall project execution. R.J.L. supervised overall project design and execution. A.K.S., G.H.W., K.X., G.S., B.K.K. and R.J.L. participated in data analysis and interpretation. A.K.S., G.H.W., K.X., G.S., B.K.K. and R.J.L. wrote the manuscript. All authors have seen and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
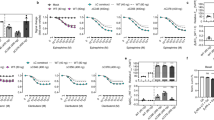
Extended Data Figure 1 Formation of the β2V2R–β-arrestin-1–Fab30 complex follows agonist occupancy of the receptor and is biochemically stable.
a, Sf9 cells co-expressing the β2V2R, β-arrestin 1 (βarr1) and GRK2CAAX were stimulated with varying doses of the high-affinity agonist BI-167107 followed by addition of Fab30 and purification of the complexes. Stimulation of cells with increasing concentration of BI-167107 results in increasing amount of β-arrestin 1 co-purification, indicating a direct correlation between occupancy of the receptor with agonist and complex formation. b, Quantification of agonist-dependent complex formation from seven independent experiments normalized with respect to the β-arrestin-1 signal at the highest agonist concentration. c, Purified T4L–β2V2R–β-arrestin-1–Fab30 complex was stored either at 4 °C or at room temperature for 4 days followed by size exclusion chromatography on a Superdex 200 (10/300) column (flow rate 0.5 ml min−1). No substantial dissociation of the complex was detected as monitored by appearance of a peak corresponding to the receptor (13.5 ml) or β-arrestin 1 (14.5 ml).
Extended Data Figure 2 Functionally relevant conformation of β-arrestin 1 in the T4L–β2V2R–β-arrestin-1–Fab30 complex as revealed by enhanced clathrin-TD interaction.
Purified glutathione S-transferase (GST)-tagged clathrin-TD was added to the purified complex or an equivalent amount of β-arrestin 1 (βarr1) alone. Interaction of clathrin-TD with the complex or β-arrestin 1 was measured by subsequent co-immunoprecipitation and western blot analysis. Quantification of four independent experiments shown as a bar graph. The relative intensities of the β-arrestin-1 bands are normalized with respect to β-arrestin 1 alone (set as 1). A Coomassie-stained gel indicating comparable amounts of β-arrestin 1 for complex versus β-arrestin 1 alone conditions in clathrin-TD co-immunoprecipitation experiments is shown on the left. Error bars show standard error of the mean (s.e.m.). P < 0.05 for paired t-test.
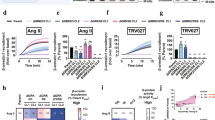
Extended Data Figure 3 HDX-MS analysis and MS-based mapping of the crosslinking site in T4L–β2V2R–β-arrestin-1–Fab30 complex.
a, The differential HDX between the T4L–β2V2R–β-arrestin-1–Fab30 complex and the V2Rpp–β-arrestin-1–Fab30 complex are mapped on the sequence of β-arrestin 1 (βarr1). b, DSA, a homobifunctional amine-reactive crosslinker, was used to crosslink the preformed T4L–β2V2R–β-arrestin-1–Fab30 complex. c, A representative SDS–PAGE showing the DSA crosslinking efficiency of the preformed complex. d, The crosslinked peptides were characterized with ‘doublet’ peak signatures in mass spectra as described in Methods and revealed a crosslink between K235 of the β2V2R and K77 at the distal end of the finger loop in β-arrestin 1. e, Structural model of the β2V2R–β-arrestin-1 complex highlighting the crosslinking site.
Extended Data Figure 4 Disulphide trapping strategy reveals close proximity of residue 235 of the β2V2R and residue 78 at the distal end of the finger loop in β-arrestin 1.
a, Structural model of the β2V2R–β-arrestin-1 complex depicting the proximity of K235 on the β2V2R and D78 on β-arrestin 1 (βarr1). b, Single cysteine insertion mutants of the β2V2R (covering residues 231–236) and β-arrestin-1(D78C) were co-transfected in HEK-293 cells and complex formation was induced by stimulating the cells with an oxidizing agent, H2O2 and agonist (isoproterenol (Iso)). Subsequently, a co-immunoprecipitation assay was performed using Flag M2 beads (Flag–β-arrestin 1). Formation of disulphide trapped complex was visualized by western blotting. c, Quantification of β-arrestin 1 in S–S trapped complex from three independent experiments with s.e.m.
Extended Data Figure 5 Raw EM images of negative-stained native T4L–β2V2R–β-arrestin-1–Fab 30/ScFv30 complex.
a, Raw EM image of T4L–β2V2R–β-arrestin-1–Fab30 complex. b, Raw EM image of T4L–β2V2R–β-arrestin-1–ScFv30 complex. Scale bar, 100 nm.
Extended Data Figure 6 Two-dimensional classifications of the T4L–β2V2R–β-arrestin-1–Fab30/ScFv30 complex.
a, b, Reference-free two-dimensional class averages were obtained using ISAC. a, Two-dimensional classification of the T4L–β2V2R–β-arrestin-1–Fab30 complex. b, Two-dimensional classification of the T4L–β2V2R–β-arrestin-1–ScFv30 complex. Scale bar, 10 nm.
Extended Data Figure 7 ‘On-column’ glutaraldehyde crosslinking of the preformed complex.
a, Schematic representation of the on-column crosslinking strategy. A glutaraldehyde solution is injected to a size exclusion chromatography column, followed by injection of the purified complex protein. As the complex protein passes through the glutaraldehyde bolus, the receptor and the β-arrestin (βarr) components of the complex are crosslinked through proximal primary amine groups. This procedure allows only brief exposure of the complex to glutaraldehyde and serves as an ‘in-line’ purification of homogenously crosslinked protein from any aggregation that may arise from non-specific crosslinking. b, On-column crosslinking of the T4L–β2V2R–β-arrestin-1–ScFv30 complex. Purified complex (approximately 20 μM) was injected onto a 24 ml Superdex 200 gel filtration column after a pre-injection of 200 μl of 0.25% glutaraldehyde bolus. Individual fractions were collected and analysed by SimplyBlue-stained SDS–PAGE. c, On-column crosslinking of the T4L–β2V2R–β-arrestin-1–Fab30 complex performed as described for the ScFv complex earlier.
Extended Data Figure 8 Raw EM images of negative-stained crosslinked T4L–β2V2R–β-arrestin-1–Fab30/ScFv30 complex.
a, Raw EM image of T4L–β2V2R–β-arrestin-1–Fab30 complex. b, Raw EM image of T4L–β2V2R–β-arrestin-1–ScFv30 complex. Scale bar, 100 nm.
Extended Data Figure 9 Two-dimensional classifications of crosslinked T4L–β2V2R–β-arrestin-1–Fab30/ScFv30 complex.
a, b, Reference-free two-dimensional class averages were obtained using ISAC. a, Two-dimensional classification of crosslinked T4L–β2V2R–β-arrestin-1–Fab30 complex. b, Two-dimensional classification of crosslinked T4L–β2V2R–β-arrestin-1–ScFv30 complex. Scale bar, 10 nm.
Extended Data Figure 10 Three-dimensional EM reconstructions and resolution indications by FSC.
The top panel shows the three-dimensional map from particles representing the fully engaged β2V2R–β-arrestin-1 conformation of the T4L–β2V2R–β-arrestin-1–Fab30 complex. The bottom panel shows the three-dimensional reconstruction from particles displaying the loose, hanging arrestin conformation of the same complex. Representative two-dimensional averages of particles used for the calculation of initial models by the random conical tilt method are shown on the left of each respective three-dimensional map.
Rights and permissions
About this article
Cite this article
Shukla, A., Westfield, G., Xiao, K. et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222 (2014). https://doi.org/10.1038/nature13430
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13430
This article is cited by
-
β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR
Nature Chemical Biology (2024)
-
Distinct activation mechanisms of β-arrestin-1 revealed by 19F NMR spectroscopy
Nature Communications (2023)
-
QR code model: a new possibility for GPCR phosphorylation recognition
Cell Communication and Signaling (2022)
-
Heterotrimeric Gq proteins act as a switch for GRK5/6 selectivity underlying β-arrestin transducer bias
Nature Communications (2022)
-
Agonist dependency of the second phase access of β-arrestin 2 to the heteromeric µ-V1b receptor
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.