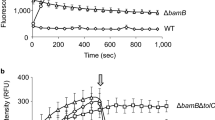
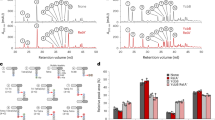
Abstract
Peptide nucleic acid (PNA) is a DNA mimic with attractive properties for developing improved gene-targeted antisense agents. To test this potential of PNA in bacteria, PNAs were designed to target the start codon regions of the Escherichia coli β-galactosidase and β-lactamase genes. Dose-dependent and specific gene inhibition was observed in vitro using low nanomolar PNA concentrations and in vivo using low micromolar concentrations. Inhibition was more efficient for a permeable E. coli strain relative to wild-type K-12. The potency of the anti-β-lactamase PNAs was abolished by a six base substitution, and inhibition could be re-established using a PNA with compensating base changes. Antisense inhibition of the β-lactamase gene was sufficient to sensitize resistant cells to the antibiotic ampicillin. The results demonstrate gene- and sequence-specific antisense inhibition in E. coli and open possibilities for anti-sense antibacterial drugs and gene function analyses in bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Monia, B.P., Johnston, J.F., Geiger, T., Muller, M., and Fabbro, D. 1996. Antitumor activity of a phosphorothioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nat. Med. 2: 668–675.
Nyce, J.W. and Metzger, W.J. 1997. DNA antisense therapy for asthma in an animal model. Nature 385: 721–725.
Simons, R.W. and Kleckner, N. 1998. Biological regulation by antisense RNA in prokaryotes. Annu. Rev. Genet 22: 567–600.
Jayaraman, K., McParland, K. Miller, P., and Ts'o, P.O. 1981. Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3′ end of 16S rRNA. Proc. Natl. Acad. Sci. USA 78: 1537–1541.
Gasparro, F.P., Edelson, R.L., O'Malley, M.E. Ugent, S.J., and Wong, H.H. 1991. Photoactivatable antisense DNA: suppression of ampicillin resistance in normally resistant Escherichia coli . Antisense Res. Dev. 1: 117–140.
Nielsen, P.E., Egholm, M., Berg, R.H., and Buchardt, O. 1991. Sequence selective recognition of DNA by strand displacement with a thyrnine-substituted polyamide. Science 254: 1497–1500.
Wittung, P., Nielsen, P.E., Buchardt, O., Egholm, M. and Nordén, B. 1994. DNA-like double helix formed by peptide nucleic acid. Nature 368: 561–563.
Egholm, M., Buchardt, O., Christensen, L., Behrens, C., Freier, S.M., Driver, D.A. et al. 1993. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen bonding rules. Nature 365: 566–568.
Demidov, V., Potaman, V.N., Frank-Kamenetskii, M.D., Buchardt, O., Egholm, M., and Nielsen, P.E. 1994. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem. Pharmacol. 48: 1309–1313.
Nielsen, P.E. and Haaima, G. 1997. Peptide nucleic acid PNA. A DNA mimic with a pseudopeptide backbone. Chem. Soc. Rev. 96: 73–78.
Hanvey, J.C., Peffer, N.J., Bisi, J.E., Thomson, S.A., Cadilla, R., Josey, J.A. et al. 1992. Antisense and antigene properties of peptide nucleic acids. Science 258: 1481–1485.
Nielsen, P.E., Egholm, M., and Buchardt, O. 1994. Sequence-specific transcription arrest by peptide nucleic acid bound to the DNA template strand. Gene 149: 139–145.
Bonham, M.A., Brown, S., Boyd, A.L., Brown, P.H., Bruckenstein, D.A., Hanvey, J.C. et al. 1995. An assessment of the antisense properties of RNase H-competent and steric-blocking oligomers. Nucl. Acids Res. 23: 1197–1203.
Knudsen, H. and Nielsen, P.E. 1996. Antisense properties of duplex-and triplex-forming PNAs. Nucl. Acids Res. 24: 494–500.
Norton, J.C., Piatyczek, J.A., Wright, W.E., Shay, J.W., and Corey, D.R. 1996. Inhibition of human telomerase activity by peptide nucleic acid. Bio/technology 14: 615–619.
Taylor, R.W., Chinnery, P.F., Turnbull, D.M. and Lightowlers, R.N. 1997. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat. Genet. 15: 212–215.
Thorson, J.S., Cornish, V.W., Barrett, J.E., Cload, S.T., Yano, T., and Schultz, P.G. A biosynthetic approach for the incorporation of unnatural amino acids into proteins. Methods Mol. Biol In press.
Sekiguchi, M. and Lida, S. 1967. Mutants of Escherichia coli permeable to actinomycin. Proc. Natl. Acad. Sci. USA 58: 2315–2320.
Wittung, P., Kajanus, J., Edwards, K., Nielsen, P., Nordén, B., and Malmström, B.G. 1995. Phospholipid membrane permeability of peptide nucleic acid. FEBS Lett. 365: 27–29.
Gardner, A.D. 1940. Morphological effects of penicillin on bacteria. Nature 146: 837–838.
Sørensen, M.A., Kurland, C.G., and Pedersen, S.J. 1989. Codon usage determines translation rate in Escherichia coli . J. Mol. Biol. 207: 365–377.
Wren, B.W., Henderson, J., and Ketley, J.M. 1994. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. Biotechniques 16: 994–996.
Christensen, L., Fitzpatrick, R., Gildea, B., Petersen, K.H., Hansen, H.F., Koch, T. et al. 1995. Solid-phase synthesis of peptide nucleic acids. Journal of Peptide Science 3: 175–183.
Miller, J.H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY.
Kobayashi, S., Arai, S., Hayashi, S. and Sakaguchi, T. 1988. Simple assay of β-lactamase with agar medium containing a chromogenic cephalosporin, pyridini-um-2-azo-p-dimethylaniline chromophore (PADAC). Antimicrob. Agents Chemother. 32: 1040–1045.
Lauer, B.A., Reller, L.B. and Mirrett, S. 1981. Comparison of acridine orange and gram strains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J. Clin. Microbiol. 14: 201–205.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Good, L., Nielsen, P. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol 16, 355–358 (1998). https://doi.org/10.1038/nbt0498-355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0498-355
This article is cited by
-
Translocation of non-lytic antimicrobial peptides and bacteria penetrating peptides across the inner membrane of the bacterial envelope
Current Genetics (2022)
-
Walking through the wonder years of artificial DNA: peptide nucleic acid
Molecular Biology Reports (2020)
-
Advances in therapeutic bacterial antisense biotechnology
Applied Microbiology and Biotechnology (2018)
-
Impact of different cell penetrating peptides on the efficacy of antisense therapeutics for targeting intracellular pathogens
Scientific Reports (2016)
-
Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression
Scientific Reports (2015)