Abstract
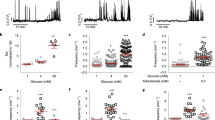
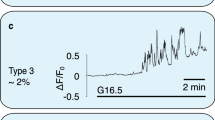
Pancreatic islets have a central role in blood glucose homeostasis. In addition to insulin-producing β-cells and glucagon-secreting α-cells, the islets contain somatostatin-releasing δ-cells1. Somatostatin is a powerful inhibitor of insulin and glucagon secretion2. It is normally secreted in response to glucose3 and there is evidence suggesting its release becomes perturbed in diabetes4. Little is known about the control of somatostatin release. Closure of ATP-regulated K+-channels (KATP-channels)5 and a depolarization-evoked increase in cytoplasmic free Ca2+ concentration ([Ca2+]i)6,7,8 have been proposed to be essential. Here, we report that somatostatin release evoked by high glucose (≥10 mM) is unaffected by the KATP-channel activator diazoxide and proceeds normally in KATP-channel-deficient islets. Glucose-induced somatostatin secretion is instead primarily dependent on Ca2+-induced Ca2+-release (CICR). This constitutes a novel mechanism for KATP-channel-independent metabolic control of pancreatic hormone secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bishop, A. E. Polak, J. M. The anatomy, organisation and ultrastructure of the islets of Langerhans. in Textbook of Diabetes, Vol. 1 (ed. Pickup, J. W.) 6.1–6.16 (Blackwell Science, Oxford, 1997).
Schuit, F. C., Derde, M. P. & Pipeleers, D. G. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia 32, 207–212 (1989).
Efendic, S., Enzmann, F., Nylen, A., Uvnäs-Wallensten, K. & Luft, R. Effect of glucose/sulfonylurea interaction on release of insulin, glucagon, and somatostatin from isolated perfused rat pancreas. Proc. Natl Acad. Sci. USA 76, 5901–5904 (1979).
Abdel-Halim, S. M., Guenifi, A., Efendic, S. & Östenson, C. G. Both somatostatin and insulin responses to glucose are impaired in the perfused pancreas of the spontaneously noninsulin-dependent diabetic GK (Goto-Kakizaki) rats. Acta. Physiol. Scand. 148, 219–226 (1993).
Göpel, S. O., Kanno, T., Barg, S. & Rorsman, P. Patch-clamp characterisation of somatostatin-secreting δ-cells in intact mouse pancreatic islets. J. Physiol. 528, 497–507 (2000).
Efendic, S., Grill, V., Nylen, A. & Östensson, C. G. Difference in calcium dependency of insulin, glucagon and somatostatin secretion in response to glibenclamide in perfused rat pancreas. Diabetologia 22, 475–479 (1982).
Quesada, I., Nadal, A. & Soria, B. Different effects of tolbutamide and diazoxide in α- β-, and δ-cells within intact islets of Langerhans. Diabetes 48, 2390–2397 (1999).
Nadal, A., Quesada, I. & Soria, B. Homologous and heterologous asynchronicity between identified α, β and δ-cells within intact islets of Langerhans in the mouse. J. Physiol. 517, 85–93 (1999).
Vieira, E., Salehi, A. & Gylfe, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic α cells. Diabetologia 50, 370–379 (2007).
Shiota, C. et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J. Biol. Chem. 277, 37176–37183 (2002).
Seghers, V., Nakazaki, M., DeMayo, F., Aguilar-Bryan, L. & Bryan, J. Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J. Biol. Chem. 275, 9270–9277 (2000).
Miki, T. et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl Acad. Sci. USA 95, 10402–10406 (1998).
Göpel, S. et al. Capacitance measurements of exocytosis in mouse pancreatic α, β- and δ-cells within intact islets of Langerhans. J. Physiol. 556, 711–726 (2004).
Fill, M. & Copello, J. A. Ryanodine receptor calcium release channels. Physiol. Rev. 82, 893–922 (2002).
Johnson, J. D. et al. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J. Biol. Chem. 279, 24794–24802 (2004).
Mitchell, K. J., Lai, F. A. & Rutter, G. A. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic β-cells (MIN6). J. Biol. Chem. 278, 11057–11064 (2003).
Islam, M. S. et al. In situ activation of the type 2 ryanodine receptor in pancreatic β cells requires cAMP-dependent phosphorylation. Proc. Natl Acad. Sci. USA 95, 6145–6150 (1998).
Rose, C. R. & Konnerth, A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron 31, 519–522 (2001).
Leiter, E. H., Gapp, D. A., Eppig, J. J. & Coleman, D. L. Ultrastructural and morphometric studies of δ cells in pancreatic islets from C57BL/Ks diabetes mice. Diabetologia 17, 297–309 (1979).
Bokvist, K., Eliasson, L., Ämmälä, C., Renstrom, E. & Rorsman, P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic β-cells. EMBO J. 14, 50–57 (1995).
Chow, R. H., Lund, P. E., Loser, S., Panten, U. & Gylfe, E. Coincidence of early glucose-induced depolarization with lowering of cytoplasmic Ca2+ in mouse pancreatic β-cells. J. Physiol. 485, 607–617 (1995).
Tengholm, A., Hellman, B. & Gylfe, E. The endoplasmic reticulum is a glucose-modulated high-affinity sink for Ca2+ in mouse pancreatic β-cells. J. Physiol. 530, 533–540 (2001).
Jing, X. et al. CaV2.3 calcium channels control second-phase insulin release. J. Clin. Invest. 115, 146–154 (2005).
Szollosi, A., Nenquin, M., Aguilar-Bryan, L., Bryan, J. & Henquin, J. C. Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old β-cells lacking ATP-sensitive K+ channels. J. Biol. Chem. 282, 1747–1756 (2007).
Quwailid, M. M. et al. A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm. Genome 15, 585–591 (2004).
Lollike, K. & Lindau, M. Membrane capacitance techniques to monitor granule exocytosis in neutrophils. J. Immunol. Methods 232, 111–120 (1999).
Kanno, T. et al. Large dense-core vesicle exocytosis in pancreatic β-cells monitored by capacitance measurements. Methods 33, 302–311 (2004).
Wendt, A. et al. Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53, 1038–1045 (2004).
Prentki, M. et al. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature 309, 562–564 (1984).
Squires, P. E., Amiranoff, B. & Dunne, M. J. Intracellular Ca2+ signals in human-derived pancreatic somatostatin-secreting cells (QGP-1N). Pflugers Arch. 428, 275–282 (1994).
Acknowledgements
We thank M. A. Magnuson (Vanderbilt University) for supplying the SUR1-deficient mice. This work is supported by the Wellcome Trust, DiabetesUK and the European Union (Biosim, LSHB-CT-2004-005137; and Eurodia, SHM-CT-2006-518153). F.M.A. is a Royal Society Research Professor and P.R. is a Royal Society Wolfson Merit Award Research Fellow.
Author information
Authors and Affiliations
Contributions
Q.Z. performed most of the electrophysiological experiments, including those involving microfluorimetry and confocal imaging. C.P. performed all immunocytochemistry. S.G. participated in the early stages of the electrophysiological experiments were also performed by M. Braun. The PCR analyses were performed by M. Bengtsson. The hormone release measurements were performed by A.S. P.O.B., R.C., E.R. and T.S. provided SUR1−/−, Kir6.2STOP, and Cav2.3−/−, respectively. P.R.V.J. provided human islet cells (data not shown). L.E. analysed δ-cell ultrastructure using electron microscopy. F.M.A. contributed to the discussion of the data throughout the study and assisted with manuscript preparation. The study was initiated by P.R. who directed all experimental protocols used, performed some of the electrophysiological measurements, liaised with all co-authors and was the main author of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 649 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Bengtsson, M., Partridge, C. et al. R-type Ca2+-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat Cell Biol 9, 453–460 (2007). https://doi.org/10.1038/ncb1563
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1563
This article is cited by
-
Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets
Cellular and Molecular Life Sciences (2023)
-
Acetyl-CoA-carboxylase 1 (ACC1) plays a critical role in glucagon secretion
Communications Biology (2022)
-
Somatostatin secretion by Na+-dependent Ca2+-induced Ca2+ release in pancreatic delta cells
Nature Metabolism (2020)
-
Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion
Nature Communications (2019)
-
Integrating the inputs that shape pancreatic islet hormone release
Nature Metabolism (2019)