Abstract
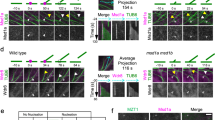
AMP-activated protein kinase (AMPK) is an energy-sensing Ser/Thr protein kinase originally shown to be regulated by AMP1. AMPK is activated by various cellular stresses that inhibit ATP production or stimulate ATP consumption2. In addition to its role in metabolism, AMPK has recently been reported to reshape cells by regulating cell polarity and division3,4,5,6. However, the downstream targets of AMPK that participate in these functions have not been fully identified. Here, we show that phosphorylation of the microtubule plus end protein CLIP-170 by AMPK is required for microtubule dynamics and the regulation of directional cell migration. Both inhibition of AMPK and expression of a non-phosphorylatable CLIP-170 mutant resulted in prolonged and enhanced accumulation of CLIP-170 at microtubule tips, and slower tubulin polymerization. Furthermore, inhibition of AMPK impaired microtubule stabilization and perturbed directional cell migration. All of these phenotypes were rescued by expression of a phosphomimetic CLIP-170 mutant. Our results demonstrate, therefore, that AMPK controls basic cellular functions by regulating microtubule dynamics through CLIP-170 phosphorylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yeh, L. A., Lee, K. H. & Kim, K. H. Regulation of rat liver acetyl-CoA carboxylase. Regulation of phosphorylation and inactivation of acetyl-CoA carboxylase by the adenylate energy charge. J. Biol. Chem. 255, 2308–2314 (1980).
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Rev. Mol. Cell Biol. 8, 774–785 (2007).
Zhang, L., Li, J., Young, L. H. & Caplan, M. J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl Acad. Sci. USA 103, 17272–17277 (2006).
Zheng, B. & Cantley, L. C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl Acad. Sci. USA 104, 819–822 (2007).
Mirouse, V., Swick, L. L., Kazgan, N., St. Johnston, D. & Brenman, J. E. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 177, 387–392 (2007).
Lee, J. H. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, 1017–1020 (2007).
Williams, T. & Brenman, J. E. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 18, 193–198 (2008).
Jansen, M., Ten Klooster, J. P., Offerhaus, G. J. & Clevers, H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol. Rev. 89, 777–798 (2009).
Martin, S. G. & St. Johnston, D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature 421, 379–384 (2003).
Watts, J. L., Morton, D. G., Bestman, J. & Kemphues, K. J. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127, 1467–1475 (2000).
Rickard, J. E. & Kreis, T. E. Identification of a novel nucleotide-sensitive microtubule-binding protein in HeLa cells. J. Cell Biol. 110, 1623–1633 (1990).
Pierre, P., Scheel, J., Rickard, J. E. & Kreis, T. E. CLIP-170 links endocytic vesicles to microtubules. Cell 70, 887–900 (1992).
Dragestein, K. A. et al. Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends. J. Cell Biol. 180, 729–737 (2008).
Hardie, D. G., Carling, D. & Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821–855 (1998).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007).
Perez, F., Diamantopoulos, G. S., Stalder, R. & Kreis, T. E. CLIP-170 highlights growing microtubule ends in vivo. Cell 96, 517–527 (1999).
Bieling, P. et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J. Cell Biol. 183, 1223–1233 (2008).
Wu, X., Kodama, A. & Fuchs, E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 135, 137–148 (2008).
Rodriguez, O. C. et al. Conserved microtubule–actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5, 599–609 (2003).
Small, J. V., Geiger, B., Kaverina, I. & Bershadsky, A. How do microtubules guide migrating cells? Nature Rev. Mol. Cell Biol. 3, 957–964 (2002).
Turner, C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2, E231–E236 (2000).
Broussard, J. A., Webb, D. J. & Kaverina, I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 20, 85–90 (2008).
Fukata, M. et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109, 873–885 (2002).
Palazzo, A. F. et al. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536–1541 (2001).
Komarova, Y. et al. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol. Biol. Cell 16, 5334–5345 (2005).
Acknowledgements
We thank M. Amano and S. Fukuhara for helpful discussions, and M. Koyama (Olympus Corporation) for technical advice regarding microscopy. This research was supported by: a Grants-in-Aid from the Ministry of Health, Labour and Welfare of Japan; Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan; grants from the Japan Heart Foundation; grants from the Japan Cardiovascular Research Foundation; a grant from the Japan Society for the Promotion of Science; a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research; and a Grant-in-Aid from the Japan Medical Association.
Author information
Authors and Affiliations
Contributions
A.N. designed and conducted the study, performed most of the experiments, and wrote the manuscript; S.T. designed and conducted the study, performed the biochemical experiments and wrote the manuscript; H.K. carried out immunoblot analysis; K.M. independently counted the number of cells; S.Y. helped to generate the plasmids; Y.A., O.S., S.H., Y.S., H.A., M.A. and T.M. discussed the results and reviewed the manuscript; T.W. and K.K. generated and provided antibodies and Vero cells and reviewed the manuscript; N.M. conducted and supported the biological experiments and wrote the manuscript; M.K. supervised all work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1180 kb)
Supplementary Information
Supplementary Movie 1 (MOV 9923 kb)
Supplementary Information
Supplementary Movie 2 (MOV 8991 kb)
Supplementary Information
Supplementary Movie 3 (MOV 10363 kb)
Supplementary Information
Supplementary Movie 4 (MOV 1498 kb)
Supplementary Information
Supplementary Movie 5 (MOV 5336 kb)
Supplementary Information
Supplementary Movie 6 (MOV 6713 kb)
Supplementary Information
Supplementary Movie 7 (MOV 11565 kb)
Supplementary Information
Supplementary Movie 8 (MOV 9470 kb)
Rights and permissions
About this article
Cite this article
Nakano, A., Kato, H., Watanabe, T. et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol 12, 583–590 (2010). https://doi.org/10.1038/ncb2060
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2060
This article is cited by
-
The autophagy inducer SMER28 attenuates microtubule dynamics mediating neuroprotection
Scientific Reports (2022)
-
Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes
Cellular & Molecular Immunology (2022)
-
Ubiquitination of CLIP-170 family protein restrains polarized growth upon DNA replication stress
Nature Communications (2022)
-
Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration
Cell Death & Disease (2022)
-
Phosphoproteomic analysis of neoadjuvant breast cancer suggests that increased sensitivity to paclitaxel is driven by CDK4 and filamin A
Nature Communications (2022)