Abstract
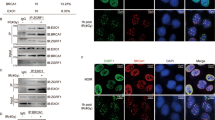
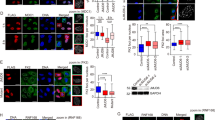
53BP1 is a conserved nuclear protein that is implicated in the DNA damage response. After irradiation, 53BP1 localizes rapidly to nuclear foci, which represent sites of DNA double strand breaks1,2,3,4, but its precise function is unclear. Using small interference RNA (siRNA), we demonstrate that 53BP1 functions as a DNA damage checkpoint protein. 53BP1 is required for at least a subset of ataxia telangiectasia-mutated (ATM)-dependent phosphorylation events at sites of DNA breaks and for cell cycle arrest at the G2–M interphase after exposure to irradiation. Interestingly, in cancer cell lines expressing mutant p53, 53BP1 was localized to distinct nuclear foci and ATM-dependent phosphorylation of Chk2 at Thr 68 was detected, even in the absence of irradiation. In addition, more than 50% of Chk2 was phosphorylated at Thr 68 in surgically resected lung and breast tumour specimens from otherwise untreated patients. We conclude that the constitutive activation of the DNA damage checkpoint pathway may be linked to the high frequency of p53 mutations in human cancer, as p53 is a downstream target of Chk2 and ATM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schultz, L. B., Chehab, N. H., Malikzay, A. & Halazonetis, T. D. J. Cell Biol. 151, 1381–1390 (2000).
Rappold, I., Iwabuchi, K., Date, T. & Chen, J. J. Cell Biol. 153, 613–620 (2001).
Anderson, L., Henderson, C. & Adachi, Y. Mol. Cell. Biol. 21, 1719–1729 (2001).
Xia, Z., Morales, J. C., Dunphy, W. G. & Carpenter, P. B. J. Biol. Chem. 276, 2708–2718 (2001).
Shiloh, Y. & Kastan, M. B. Adv. Cancer Res. 83, 209–254 (2001).
Weinert, T. A. & Hartwell, L. H. Science 241, 317–322 (1988).
Vialard, J. E., Gilbert, C. S., Green, C. M. & Lowndes, N. F. EMBO J. 17, 5679–5688 (1998).
Sun, Z., Hsiao, J., Fay, D. S. & Stern, D. F. Science 281, 272–274 (1998).
Schwartz, M. F., Duong, J. K., Sun, Z., Morrow, J. S., Pradhan, D. & Stern, D. F. Mol. Cell 9, 1055–1065 (2002).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. J. Biol. Chem. 273, 5858–5868 (1998).
Rogakou, E. P., Boon, C., Redon, C. & Bonner, W. M. J. Cell Biol. 146, 905–916 (1999).
Abraham, R. T. Genes Dev. 15, 2177–2196 (2001).
Kim, S. T., Xu, B. & Kastan, M. B. Genes Dev. 16, 560–570 (2002).
Yazdi, P. T., Wang, Y., Zhao, S., Patel, N., Lee, E. Y. & Qin, J. Genes Dev. 16, 571–582 (2002).
Andegeko, Y., Moyal, L., Mittelman, L., Tsarfaty, I., Shiloh, Y. & Rotman, G. J. Biol. Chem. 276, 38224–38230 (2001).
Xu, B., Kim, S. T., Lim, D. S. & Kastan, M. B. Mol. Cell. Biol. 22, 1049–1059 (2002).
Vogelstein, B., Lane, D. & Levine, A. J. Nature 408, 307–310 (2000).
Bartek, J., Falck, J. & Lukas, J. Nature Rev. Mol. Cell. Biol. 2, 877–886 (2001).
McGowan, C. H. Bioessays 24, 502–511 (2002).
Bell, D. W. et al. Science 286, 2528–2531 (1999).
Meijers-Heijboer, H. et al. Nature Genet. 31, 55–59 (2002).
Lukas, C. et al. Cancer Res. 61, 4990–4993 (2001).
Boulton, S. J. et al. Science 295, 127–131 (2002).
Saka, Y., Esashi, F., Matsusaka, T., Mochida, S. & Yanagida, M. Genes Dev. 11, 3387–3400 (1997).
Wang, B., Matsuoka, S., Carpenter, P. B. & Elledge, S. J. Science online (cited 3 October 2002) 10.1126-science.1076182 (2002).
Bartkova, J et al. Cancer Res. 55, 949–956 (1995).
Falck, J., Mailand, N., SyljuÂsen, R. G., Bartek, J. & Lukas, J. Nature 410, 842–847 (2001).
Acknowledgements
We thank J. Chen, S. J. Elledge, A. Nussenzweig and Y. Shiloh for helpful discussions and communication of unpublished observations. We also thank L. Schultz for her early contribution to this project. This work was supported by grants CA76367 and CA25874 (T.D.H.) and CA09171 (Wistar Institute Training Grant - T.A.M.) from the National Cancer Institute and by grants from the Danish Cancer Society and the European Commission (J.B., M.S. and J.B.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Figure S1 Suppression of 53BP1 protein levels by siRNA does not affect the cell cycle profile of non-irradiated cells. (PDF 116 kb)
Figure S2 Suppression of 53BP1 protein levels by siRNA does not affect the γ-H2AX foci present in non-irradiated SAOS2 cells.
Figure S3 Specificity of the immunohistochemistry assay for Chk2 phosphorylation at Thr68.
Rights and permissions
About this article
Cite this article
DiTullio, R., Mochan, T., Venere, M. et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4, 998–1002 (2002). https://doi.org/10.1038/ncb892
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb892
This article is cited by
-
Changes in calpain-2 expression during glioblastoma progression predisposes tumor cells to temozolomide resistance by minimizing DNA damage and p53-dependent apoptosis
Cancer Cell International (2023)
-
Combined inhibition of EZH2 and ATM is synthetic lethal in BRCA1-deficient breast cancer
Breast Cancer Research (2022)
-
JMJD6 modulates DNA damage response through downregulating H4K16ac independently of its enzymatic activity
Cell Death & Differentiation (2020)
-
Distinct associations of the Saccharomyces cerevisiae Rad9 protein link Mac1-regulated transcription to DNA repair
Current Genetics (2020)
-
Radiation-dose-dependent functional synergisms between ATM, ATR and DNA-PKcs in checkpoint control and resection in G2-phase
Scientific Reports (2019)