Abstract
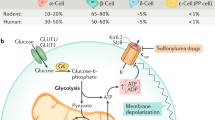
Homeostasis of blood glucose is maintained by hormone secretion from the pancreatic islets of Langerhans. Glucose stimulates insulin secretion from β-cells but suppresses the release of glucagon, a hormone that raises blood glucose, from α-cells1. The mechanism by which nutrients stimulate insulin secretion has been studied extensively: ATP has been identified as the main messenger and the ATP-sensitive potassium channel as an essential transducer in this process2. By contrast, much less is known about the mechanisms by which nutrients modulate glucagon secretion. Here we use conventional pancreas perfusion and a transcriptional targeting strategy to analyse cell-type-specific signal transduction and the relationship between islet α- and β-cells. We find that pyruvate, a glycolytic intermediate and principal substrate of mitochondria, stimulates glucagon secretion. Our analyses indicate that, although α-cells, like β-cells, possess the inherent capacity to respond to nutrients, secretion from α-cells is normally suppressed by the simultaneous activation of β-cells. Zinc released from β-cells may be implicated in this suppression. Our results define the fundamental mechanisms of differential responses to identical stimuli between cells in a microorgan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Unger, R.H. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 8, 574–578 (1985).
Maechler, P. & Wollheim, C.B. Mitochondrial function in normal and diabetic β-cells. Nature 414, 807–812 (2001).
Baetens, D., Malaisse-Lagae, F., Perrelet, A. & Orci, L. Endocrine pancreas: three-dimensional reconstitution shows two types of islets of Langerhans. Science 206, 1323–1325 (1979).
Ishihara, H., Wang, H., Drewes, L.R. & Wollheim, C.B. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in β cells. J. Clin. Invest. 104, 1621–1629 (1999).
Bokvist, K. et al. Characterization of sulfonylurea and ATP-regulated K+ channels in rat pancreatic α cells. Pflugers Arch. 438, 428–436 (1999).
Gromada, J. et al. Adrenaline stimulates glucagon secretion in pancreatic α-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J. Gen. Physiol. 110, 217–228 (1997).
Nettelbeck, D.M., Jerome, V. & Muller, R. Gene therapy: designer promoters for tumour targeting. Trends Genet. 16, 174–181 (2000).
Pipeleers, D.G., Schuit, F.C., Van Schravendijk, C.F.H. & Van De Winkel, M. Interplay of nutrient and hormones in the regulation of glucagon release. Endocrinology 117, 817–823 (1985).
Samols, E. & Stagner, J.I. Intra-islet cell–cell interactions and insulin secretion. Diabetes Rev. 4, 207–223 (1998).
Sato, Y. et al. Enhanced and specific gene expression via tissue-specific production of Cre recombinase using adenovirus vector. Biochem. Biophys. Res. Commun. 224, 455–462 (1998).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transformants with a novel eukaryotic vector. Gene 108, 193–200 (1991).
Bjaaland, T., Jones, P.M. & Howell, S.L. Role of intracellular mediators in glucagon secretion: studies using intact and electrically permeabilized rat islets of Langerhans. J. Mol. Endocr. 1, 171–178 (1988).
Greenbaum, C.J., Havel, P.J., Taborsky, Jr., G.J. & Klaff, L.J. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J. Clin. Invest. 88, 767–773 (1991).
Rorsman, P. et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341, 233–236 (1989).
Tang, X. & Shay, N.F. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol 3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J. Nutr. 131, 1414–1420 (2001).
Vogt, K., Mellor, J., Tong, G. & Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187–196 (2001).
Grodsky, G.M., Epstein, G.H., Fanska, R. & Karam, J.H. Pancreatic action of the sulfonylureas. Fed.Proc. 36, 2714–2719 (1977).
Goepel, S.O., Kanno, S., Barg, S., Weng, X.-G., Gromada, J. & Rorsman, P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J. Physiol. (Lond.) 528, 508–520 (2000).
Barg, S., Galvanovskis, J., Goepel, S.O., Rorsman, P. & Eliasson, L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α cells. Diabetes 49, 1500–1510 (2000).
Quesada, I., Nadal, A. & Soria, B. Different effects of tolbutamide and diazoxide in α, β, and ? cells within intact islets of Langerhans. Diabetes 48, 2390–2397 (1999).
Foster, M.C., Leapman, R.D., Li, M.X. & Atwater, I. Elementary composition of secretory granules in pancreatic islets of Langerhans. Biophy. J. 64, 525–532 (1993).
Qian, W.J. et al. Detection of secretion from single pancreatic β-cells using extracellular fluorogenic reactions and confocal fluorescence microscopy. Anal. Chem. 72, 711–717 (2000).
Hurley, L.S. Zinc deficiency in the developing rat. Am. J. Clin. Nutr. 22, 1669–1677 (1969).
Lakso, M. et al. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl Acad. Sci. USA 89, 6232–6236 (1992).
Palmiter, R.D. et al. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50, 435–443 (1987).
Miyake, S. et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl Acad. Sci. USA 93, 1320–1324 (1996).
Gerber, P.P., Trimble, E.R., Wollheim, C.B., Renold, A.E. & Miller, R.E. Glucose and cyclic AMP as stimulators of somatostatin and insulin secretion from the isolated, perfused rat pancreas: a quantitative study. Diabetes 30, 40–44 (1981).
Herrera, P.L. et al. Embriogenesis of the murine pancreas: early expression of pancreatic polypeptide gene. Development 113, 1257–1265 (1991).
Acknowledgements
We thank O. Dupont for technical assistance. H.I. is a recipient of a fellowship from Manpei Suzuki Foundation for Diabetes Research and a fellowship from the Juvenile Diabetes Foundation International and P.M. is a fellow of the Professor Max Cloetta Foundation. This work was supported by grants from the Swiss National Science Foundation (to C.B.W., P.M. and P.L.H.) and the Juvenile Diabetes Research Foundation (to P.L.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Figure S1
Strategy to study specific cell types in heterogeneous cell populations. (PPT 360 kb)
Figure S2
Cytosolic and mitochondrial Ca2+ concentrations in response to varying concentrations of KCl in islet α- and β-cells. (PPT 362 kb)
Figure S3
Recombinant adenovirus system for expressing diphtheria toxin A (DTA) chain. (PPT 18 kb)
Rights and permissions
About this article
Cite this article
Ishihara, H., Maechler, P., Gjinovci, A. et al. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5, 330–335 (2003). https://doi.org/10.1038/ncb951
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb951
This article is cited by
-
Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases
Nature Reviews Endocrinology (2023)
-
Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets
Cellular and Molecular Life Sciences (2023)
-
Angiopoietins stimulate pancreatic islet development from stem cells
Scientific Reports (2021)
-
Integrating the inputs that shape pancreatic islet hormone release
Nature Metabolism (2019)
-
Zinc and its regulators in pancreas
Inflammopharmacology (2019)