Abstract
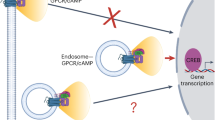
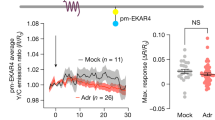
G protein–coupled receptors (GPCRs) are well known to signal via cyclic AMP (cAMP) production at the plasma membrane, but it is now clear that various GPCRs also signal after internalization. Apart from its temporal impact through prolonging the cellular response, we wondered whether the endosome-initiated signal encodes any discrete spatial information. Using the β2-adrenoceptor (β2-AR) as a model, we show that endocytosis is required for the full repertoire of downstream cAMP-dependent transcriptional control. Next, we describe an orthogonal optogenetic approach to definitively establish that the location of cAMP production is indeed the critical variable determining the transcriptional response. Finally, our results suggest that this spatial encoding scheme helps cells functionally discriminate chemically distinct β2-AR ligands according to differences in their ability to promote receptor endocytosis. These findings reveal a discrete principle for achieving cellular signaling specificity based on endosome-mediated spatial encoding of intracellular second messenger production and ′location-aware′ downstream transcriptional control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Calebiro, D. et al. Persistent cAMP-signals triggered by internalized G-protein–coupled receptors. PLoS Biol. 7, e1000172 (2009).
Ferrandon, S. et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 (2009).
Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 (2013).
Kotowski, S.J., Hopf, F.W., Seif, T., Bonci, A. & von Zastrow, M. Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290 (2011).
Lohse, M.J. & Calebiro, D. Cell biology: receptor signals come in waves. Nature 495, 457–458 (2013).
Violin, J.D. et al. β2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 283, 2949–2961 (2008).
Fan, F. et al. Novel genetically encoded biosensors using firefly luciferase. ACS Chem. Biol. 3, 346–351 (2008).
Rajagopal, S. et al. Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 80, 367–377 (2011).
Zhang, X. et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 102, 4459–4464 (2005).
Harper, C.B., Popoff, M.R., McCluskey, A., Robinson, P.J. & Meunier, F.A. Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors. Trends Cell Biol. 23, 90–101 (2013).
O′Brien, R.M., Printz, R.L., Halmi, N., Tiesinga, J.J. & Granner, D.K. Structural and functional analysis of the human phosphoenolpyruvate carboxykinase gene promoter. Biochim. Biophys. Acta 1264, 284–288 (1995).
Waxman, J.S., Hocking, A.M., Stoick, C.L. & Moon, R.T. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development 131, 5909–5921 (2004).
Zhang, L. et al. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306, 114–117 (2004).
Daaka, Y. et al. Essential role for G protein–coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 273, 685–688 (1998).
Shenoy, S.K. et al. β-arrestin-dependent, G protein–independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 (2006).
Seamon, K.B., Padgett, W. & Daly, J.W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 78, 3363–3367 (1981).
Pippig, S., Andexinger, S. & Lohse, M.J. Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 47, 666–676 (1995).
Puthenveedu, M.A. & von Zastrow, M. Cargo regulates clathrin-coated pit dynamics. Cell 127, 113–124 (2006).
Lauffer, B.E. et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565–574 (2010).
Johnson, L.S., Dunn, K.W., Pytowski, B. & McGraw, T.E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor′s internalization motif. Mol. Biol. Cell 4, 1251–1266 (1993).
Presley, J.F., Mayor, S., McGraw, T.E., Dunn, K.W. & Maxfield, F.R. Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J. Biol. Chem. 272, 13929–13936 (1997).
Stierl, M. et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 286, 1181–1188 (2011).
Kasahara, K. et al. Trafficking of Lyn through the Golgi caveolin involves the charged residues on αE and αI helices in the kinase domain. J. Cell Biol. 165, 641–652 (2004).
Gillooly, D.J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000).
Young, R.M., Holowka, D. & Baird, B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J. Biol. Chem. 278, 20746–20752 (2003).
Goldstein, D.S., Eisenhofer, G. & Kopin, I.J. Sources and significance of plasma levels of catechols and their metabolites in humans. J. Pharmacol. Exp. Ther. 305, 800–811 (2003).
Swaminath, G. et al. Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 279, 686–691 (2004).
Choy, R.W. et al. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron 82, 55–62 (2014).
Cosker, K.E. & Segal, R.A. Neuronal signaling through endocytosis. Cold Spring Harb. Perspect. Biol. 6, a020669 (2014).
Murphy, J.E., Padilla, B.E., Hasdemir, B., Cottrell, G.S. & Bunnett, N.W. Endosomes: a legitimate platform for the signaling train. Proc. Natl. Acad. Sci. USA 106, 17615–17622 (2009).
Galandrin, S., Oligny-Longpre, G. & Bouvier, M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 28, 423–430 (2007).
Taylor, S.S., Zhang, P., Steichen, J.M., Keshwani, M.M. & Kornev, A.P. PKA: lessons learned after twenty years. Biochim. Biophys. Acta 1834, 1271–1278 (2013).
Sample, V. et al. Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat. Chem. Biol. 8, 375–382 (2012).
Pontier, S.M. et al. Cholesterol-dependent separation of the β2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J. Biol. Chem. 283, 24659–24672 (2008).
Perino, A., Ghigo, A., Scott, J.D. & Hirsch, E. Anchoring proteins as regulators of signaling pathways. Circ. Res. 111, 482–492 (2012).
Ostrom, R.S., Bogard, A.S., Gros, R. & Feldman, R.D. Choreographing the adenylyl cyclase signalosome: sorting out the partners and the steps. Naunyn Schmiedebergs Arch. Pharmacol. 385, 5–12 (2012).
Kholodenko, B.N. & Kolch, W. Giving space to cell signaling. Cell 133, 566–567 (2008).
Temkin, P. et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 13, 715–721 (2011).
Burgess, A. et al. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 107, 12564–12569 (2010).
Backes, C. et al. GeneTrail—advanced gene set enrichment analysis. Nucleic Acids Res. 35, W186–W192 (2007).
Saldanha, A.J. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004).
Acknowledgements
We thank P. Brown for generously providing access to microarray facilities; P. Hegemann, H. Stenmark and T. Meyer for plasmids; D. Udwari and T. Oertner for initially suggesting the use of bPAC; and H. Bourne, B. Cheyette, R. Irannejad, B. Lobingier, A. Marley and D. Riordan for valuable discussion. These studies were supported by the National Institute on Drug Abuse of the US National Institutes of Health (DA010711 and DA012864 to M.v.Z.). N.G.T. is supported by the American Heart Association.
Author information
Authors and Affiliations
Contributions
N.G.T. performed the experiments and analyzed the data. N.G.T. and M.v.Z. designed the study, interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–4 and Supplementary Figures 1–5. (PDF 1181 kb)
Rights and permissions
About this article
Cite this article
Tsvetanova, N., von Zastrow, M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10, 1061–1065 (2014). https://doi.org/10.1038/nchembio.1665
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1665
This article is cited by
-
Cardiac contraction and relaxation are regulated by distinct subcellular cAMP pools
Nature Chemical Biology (2024)
-
Endosome positioning coordinates spatially selective GPCR signaling
Nature Chemical Biology (2024)
-
Fluorescence resonance energy transfer (FRET) spatiotemporal mapping of atypical P38 reveals an endosomal and cytosolic spatial bias
Scientific Reports (2023)
-
β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR
Nature Chemical Biology (2023)
-
Atomic force microscopy-single-molecule force spectroscopy unveils GPCR cell surface architecture
Communications Biology (2022)