Abstract
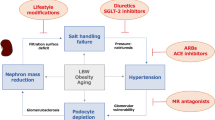
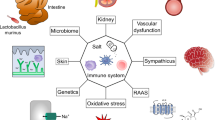
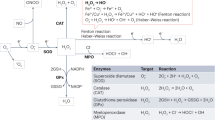
Animal studies have shown that oxidative stress and renal tubulointerstitial inflammation are associated with, and have major roles in, the pathogenesis of hypertension. This view is supported by the observations that alleviation of oxidative stress and renal tubulointerstitial inflammation reduce arterial pressure in animal models. Conversely, hypertension has been shown to cause oxidative stress and inflammation in renal and cardiovascular tissues in experimental animals. Taken together, these observations indicate that oxidative stress, inflammation and arterial hypertension participate in a self-perpetuating cycle which, if not interrupted, can lead to progressive cardiovascular disease and renal complications. These events usually occur in an insidious and asymptomatic manner over an extended period following the onset of hypertension. Severe target organ injury can, however, occasionally occur precipitously in the course of malignant or accelerated hypertension. Given the high degree of heterogeneity of hypertensive disorders, the factor(s) initiating the vicious cycle described vary considerably in different forms of hypertension. For instance, oxidative stress in the kidney and vascular tissue is the primary mediator in the pathogenesis of angiotensin-induced, and perhaps lead-induced, hypertension. By contrast, increased arterial pressure is probably the initiating trigger in salt-sensitive hypertension. Although the initiating factor might vary between hypertensive disorders, according to the proposed model, the three components of the cycle eventually coalesce in all forms of hypertension.
Key Points
-
Oxidative stress, inflammation and arterial hypertension participate in a self-perpetuating cycle that can lead to progressive cardiovascular and renal disease
-
The initiating pathogenic factor in this cycle differs in different forms of hypertension
-
Therapeutic strategies should aim to reduce production of reactive oxygen species rather than involving mere administration of antioxidant agents
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Health Organization, International Society of Hypertension Writing Group (2003) World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 21: 1983–1992
Mueller CF et al. (2005) ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278
Sen CK (2001) Antioxidant and redox regulation of cellular signaling: introduction. Med Sci Sports Exerc 33: 368–370
Taniyama Y and Griendling KK (2003) Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075–1081
Griendling KK et al. (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501
Chabrashvili T et al. (2002) Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274
Griendling KK (2004) Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493
Lassegue B and Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297
Hehner SP et al. (2000) Enhancement of T cell receptor signaling by mild oxidative shift in the intracellular thiol pool. J Immunol 165: 4319–4328
Los M et al. (1995) Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol 25: 159–165
Sen CK and Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10: 709–720
Gorman AM et al. (1999) Antioxidant-mediated inhibition of the heat shock response leads to apoptosis. FEBS Lett 445: 98–102
Kol A et al. (1999) Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 103: 571–577
Polla BS et al. (1998) Stress proteins in inflammation. Ann NY Acad Sci 851: 75–85
Landmesser U et al. (2002) Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515
Ruiz-Ortega M et al. (2000) Angiotensin II activates nuclear transcription factor κB through AT1 and AT2 receptors in cultured vascular smooth muscle cells: molecular mechanisms. Circ Res 23: 1266–1272
Egido J (1996) Vasoactive hormones and renal sclerosis. Kidney Int 49: 578–597
Wolf G and Nielson EG (1993) Angiotensin II as a renal growth factor. J Am Soc Nephrol 3: 1531–1540
Diet F et al. (1996) Increased accumulation of tissue ACE in human atherosclerotic coronary artery tissue. Circulation 94: 2756–2767
Okamura A et al. (1999) Upregulation of renin-angiotensin system during differentiation from monocytes to macrophages. J Hypertens 17: 537–545
Rodríguez-Iturbe B et al. (2004) Oxidative stress, renal infiltration of immune cells and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: 606–616
Wang D et al. (2003) Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol 14: 2783–2789
Wang D et al. (2004) Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442
Ichihara A et al. (2004) Renal renin-angiotensin system. Contrib Nephrol 143: 117–130
Navar LG (2004) The intrarenal renin-angiotensin system in hypertension. Kidney Int 65: 1522–1532
Navar LG et al. (2002) Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322
Navar LG and Nishiyama A (2004) Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115
Vanegas V (2005) Hypertension in Page (cellophane wrapped) kidney is due to interstitial nephritis. Kidney Int 68: 1161–1170
Kitiyakara C et al. (2003) Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782
Nickenig G et al. (2000) Negative feedback regulation of reactive oxygen species on AT1 receptor gene expression. Br J Pharmacol 131: 795–803
Wilcox CS (2005) Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: 913–935
Cai H and Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: role of oxidant stress. Circ Res 87: 840–844
Forstermann U and Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714
Sydow K and Munzel T (2003) ADMA and oxidative stress. Atheroscler (Suppl 4): 41–51
Kunsch C and Medford RM (1999) Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85: 753–766
Schnackenberg CG (2002) Physiological and pathophysiological roles of oxygen radicals in the renal vasculature. Am J Physiol Regul Physiol 282: R335–R342
Takahashi K et al. (1992) Glomerular actions of a free radical generated novel prostaglandin, 8-epi-prostaglandin F2 alpha: evidence for interaction with thromboxane A2 receptors. J Clin Invest 90: 136–141
Kahler J et al. (2001) Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 38: 49–57
Touyz RM (2005) Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 7: 1302–1314
Meneton P et al. (2005) Links between dietary salt intake, renal salt handling, blood pressure and cardiovascular diseases. Physiol Rev 85: 679–715
Vaziri ND et al. (2002) Pathogenesis of lead-induced hypertension: role of oxidative stress. J Hypertension 20: S15–S20
Vaziri ND et al. (1999) Increased nitric oxide inactivation by reactive oxygen species in lead-induced hypertension. Kidney Int 56: 1492–1498
Zalba G et al. (2001) Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol Dial Transplant 16 (Suppl 1): 2–5
Vaziri ND et al. (2003) Superoxide dismutase, catalase, glutathione peroxidase and NAD(P)H oxidase in lead-induced hypertension. Kidney Int 63: 186–194
Roberts CK et al. (2002) Effect of diet and exercise intervention on blood pressure, oxidative stress and nitric oxide availability. Circulation 106: 2530–2532
Roberts CK et al. (2000) Enhanced NO inactivation induced by a high fat, refined-carbohydrate diet. Hypertension 36: 423–429
Koo JR and Vaziri ND (2003) Effect of diabetes insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int 63: 195–201
Schnackenberg CG et al. (1998) Normalization of blood pressure and renal vascular resistance in SHR with a membrane permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64
Schnackenberg CG and Wilcox CS (2003) Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin f2 alpha. Hypertension 33: 424–428
Vaziri ND et al. (2000) Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 36: 957–964
Chen X et al. (2001) Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38: 606–611
Hong HJ et al. (2001) Supplementation with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38: 1044–1048
Nava M et al. (2003) Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol 284: 447–454
Rodríguez-Iturbe B et al. (2003) Antioxidant-rich diet improves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 41: 341–346
Zahn DC et al. (2004) Upregulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int 65: 219–227
Beswick RA et al. (2004) NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111
Beswick RA et al. (2001) Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786
Meng S et al. (2002) Superoxide dismutase and oxidative stress in Dahl salt-sensitive and resistant rats. Am J Physiol Regul Integr Comp Physiol 283: R732–R738
Lenda DM and Boegehold MA (2002) Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H385–H402
Zhang Y et al. (2004) The antioxidant tempol prevents and partially reverses dexamethasone-induced hypertension in the rat. Am J Hypertens 17: 260–265
Vaziri ND et al. (1998) Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. Kidney Int 53: 1748–1754
Vaziri ND et al. (2002) Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in chronic renal insufficiency. Hypertension 39: 135–141
Vaziri ND et al. (2003) Oxidative stress and dysregulation of superoxide dismutase, NADPH oxidase and xanthine oxidase in renal insufficiency. Kidney Int 63: 179–185
Heitzer T et al. (1999) Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int 55: 252–260
Dobrian AD et al. (2001) Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension 38: 361–366
Barton CH et al. (2001) Enhanced nitric oxide inactivation in aortic coarctation-induced hypertension. Kidney Int 60: 1083–1087
Vaziri ND et al. (2000) Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 36: 142–146
Zhou XJ et al. (2002) Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. J Pharmacol Exp Ther 300: 762–767
Ni Z et al. (2004) Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int 66: 2329–2336
Vaziri ND (2004) Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol 24: 469–473
Roberts CK et al. (2005) A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol 98: 203–210
Roberts CK et al. (2006) Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 55: 928–934
Sindhu RK et al. (2005) Effects of aortic coarctation on aortic antioxidant enzymes and NADPH oxidase protein expression. Life Sci 7: 945–953
Vaziri ND and Ni Z (2005) Expression of NOX-I, gp91phox, p47phox and P67phox in the aorta segments above and below coarctation. Biochim Biophys Acta 1723: 321–327
Mazzali M et al. (2003) Microvascular and tubulointerstitial injury associated with chronic hypoxia-induced hypertension. Kidney Int 63: 2088–2093
Tsukahara H et al. (2000) Increased oxidative stress in rats with chronic nitric oxide depletion: measurement of urinary 8-hydroxy-2′-deoxyguanosine excretion. Redox Rep 5: 23–28
Duarte J et al. (2002) Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J Hypertens 20: 1843–1854
Landmesser U et al. (2002) Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078
Rey FE et al. (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414
Ungvari Z et al. (2004) Chronic high pressure-induced arterial oxidative stress. Am J Pathol 165: 219–226
McNally JS et al. (2003) Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297
Hishikawa K et al. (1997) Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res 81: 797–803
Lacy F et al. (2000) Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884
Dixon LJ et al. (2005) Increased superoxide production in hypertensive patients with diabetes mellitus: role of nitric oxide synthase. Am J Hypertens 18: 839–843
Roberts CK et al. (2006) Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9 and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol 100: 1657–1665
Roggensack AM et al. (1999) Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension 33: 83–89
Duffy SJ et al. (1999) Treatment of hypertension with ascorbic acid. Lancet 354: 2048–2049
Nava M et al. (2003) Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol 284: F447–F454
Müller DN et al. (2000) NF-κB inhibition ameliorates angiotensin II-induced renal damage in rats. Hypertension 35: 193–201
Alvarez V et al. (2002) Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 283: F1132–F1141
Beswick RA et al. (2001) Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786
Ding Y et al. (2001) Lead-induced hypertension. III. Increased hydroxyl radical production. Am J Hypertens 14: 169–173
Hong HJ et al. (2000) Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol 131: 631–637
Müller DN et al. (2002) Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693
Park JB et al. (2002) Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens 15: 78–84
Quiroz Y et al. (2001) Mycophenolate mofetil prevents the salt-sensitive hypertension resulting from short-term nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47
Rodríguez-Iturbe B et al. (2005) Early and sustained inhibition of nuclear factor kappa B prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 315: 51–57
Rodríguez-Iturbe B et al. (2001) Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232
Rodríguez-Iturbe B et al. (2002) Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201
Rodríguez-Iturbe B et al. (2003) Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 41: 341–346
Tian N et al. (2005) Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension 45: 934–939
Rodríguez-Iturbe B et al. (2005) Hypertension induced by aortic coarctation above the renal arteries is associated with immune cell infiltration of the kidneys. Am J Hypertens 18: 1449–1456
Rodríguez-Iturbe B et al. (2004) Evolution of renal interstitial inflammation and NF-κB activation in spontaneously hypertensive rats. Am J Nephrol 24: 587–594
Kimmelstiel P and Wilson C (1936) Benign and malignant hypertension and nephrosclerosis: a clinical and pathological study. Am J Pathol 12: 45–81
Caetano EP et al. (1999) The clinical diagnosis of hypertensive nephrosclerosis—how reliable is it? Nephrol Dial Transplant 14: 288–290
Cannon PJ et al. (1966) Hyperuricemia in primary and renal hypertension. N Engl J Med 275: 457–464
Curtis JJ et al. (1988) Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med 85: 134–138
Kuster G and Ritz E (1989) Analgesic abuse and hypertension. Lancet 2: 1105
Weiss S and Parker F (1939) Pyelonephritis: its relation to vascular lesions and to arterial hypertension. Medicine 18: 221–315
Caimi et al. (2000) Polymorphonuclear integrins, membrane fluidity, and cytosolic Ca2 content after activation in essential hypertension. Hypertension 36: 813–817
Dorffel Y et al. (1999) Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 34: 113–117
Suematsu M et al. (2002) The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation 9: 259–276
Mills PJ et al. (2003) Immune cell CD62 L and CD11a expression in response to a psychological stressor in human hypertension. Brain Behav Immun 17: 260–267
Boshtam M et al. (2002) Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitamin Nutr Res 72: 309–314
Duffy SJ et al. (2002) Prevention of hypertension, insulin resistance and oxidative stress by α-lipoic acid. Hypertension 39: 303–307
Fotherby MD et al. (2000) Effect of vitamin C on ambulatory blood pressure and plasma lipids in older patients. J Hypertens 18: 411–415
Mullan B et al. (2002) Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40: 804–809
Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto miocardico (GISSI) (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI Prevenzione trial. Lancet 354: 447–455
Heart Protection Study Collaborative Group (2002) MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 360: 23–33
Heart Outcomes Prevention Evaluation (HOPE) investigators (2000) Vitamin E supplementation and cardiovascular events in high risk patients. N Engl J Med 342: 154–160
Stephens NG et al. (1996) Randomized controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 347: 781–786
Albanes D et al. (1996) Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 6: 1560–1570
Lee DH et al. (2004) Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr 80: 1194–1200
Roberts CK and Barnard RJ (2006) Effects of exercise and diet on chronic disease. J Appl Physiol 98: 3–30
Kojda G and Hambrecht R (2005) Molecular mechanisms of vascular adaptations to exercise: physical activity as an effective antioxidant therapy. Cardiovasc Res 67: 187–197
Sironi L et al. (2004) Anti-inflammatory effects of AT1 receptor blockade provide end-organ protection in stroke-prone rats independently from blood pressure fall. J Pharmacol Exp Ther 311: 989–995
Acknowledgements
Dr Vaziri's work was partially supported by a grant from Heart, Lung and Blood Institute (H27485). Research in Dr Rodríguez-Iturbe's laboratory is supported by FONACIT Grant F-2005000283.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vaziri, N., Rodríguez-Iturbe, B. Mechanisms of Disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Rev Nephrol 2, 582–593 (2006). https://doi.org/10.1038/ncpneph0283
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0283
This article is cited by
-
Differences between cultured astrocytes from neonatal and adult Wistar rats: focus on in vitro aging experimental models
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Effects of antioxidants on diabetic kidney diseases: mechanistic interpretations and clinical assessment
Chinese Medicine (2023)
-
Hordeum vulgare ethanolic extract mitigates high salt-induced cerebellum damage via attenuation of oxidative stress, neuroinflammation, and neurochemical alterations in hypertensive rats
Metabolic Brain Disease (2023)
-
The association between dietary total antioxidant capacity and odds and severity of irritable bowel syndrome among Iranian adults: a cross-sectional study
BMC Gastroenterology (2022)
-
Magnesium hexacyanoferrate nanocatalysts attenuate chemodrug-induced cardiotoxicity through an anti-apoptosis mechanism driven by modulation of ferrous iron
Nature Communications (2022)