Abstract
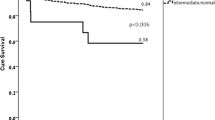
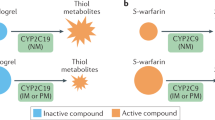
Clinical efficacy of the antiplatelet drug clopidogrel is hampered by its variable biotransformation into the active metabolite1,2. The variability in the clinical response to clopidogrel treatment has been attributed to genetic factors, but the specific genes and mechanisms underlying clopidogrel bioactivation remain unclear. Using in vitro metabolomic profiling techniques, we identified paraoxonase-1 (PON1) as the crucial enzyme for clopidogrel bioactivation, with its common Q192R polymorphism determining the rate of active metabolite formation. We tested the clinical relevance of the PON1 Q192R genotype in a population of individuals with coronary artery disease who underwent stent implantation and received clopidogrel therapy. PON1 QQ192 homozygous individuals showed a considerably higher risk than RR192 homozygous individuals of stent thrombosis, lower PON1 plasma activity, lower plasma concentrations of active metabolite and lower platelet inhibition. Thus, we identified PON1 as a key factor for the bioactivation and clinical activity of clopidogrel. These findings have therapeutic implications and may be exploited to prospectively assess the clinical efficacy of clopidogrel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
21 December 2010
In the version of this article originally published online, the affiliations for Hans-Günther Schmalz and Dirk Taubert appeared incorrectly. Hans-Günther Schmalz is in the Department für Chemie, Universität zu Köln, Cologne, Germany, and Dirk Taubert is in the Department of Pharmacology, University Hospital of Cologne, Cologne, Germany. These errors have been corrected for the print, PDF and HTML versions of the article.
07 September 2011
In the first paragraph on page 112, the authors made a typographical error: "constitutes part the active histidine dyad" should have been "is proximate to the active histidine dyad."
References
Gurbel, P.A., Antonino, M.J. & Tantry, U.S. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin. Drug Metab. Toxicol. 5, 989–1004 (2009).
Angiolillo, D.J. & Ferreiro, J.L. Platelet adenosine diphosphate P2Y12 receptor antagonism: benefits and limitations of current treatment strategies and future directions. Rev. Esp. Cardiol. 63, 60–76 (2010).
Serebruany, V.L. et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 45, 246–251 (2005).
Sofi, F. et al. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. Thromb. Haemost. 103, 841–848 (2010).
Shuldiner, A.R. et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. J. Am. Med. Assoc. 302, 849–857 (2009).
Hulot, J.-S. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J. Am. Coll. Cardiol. 56, 134–143 (2010).
Holmes, D.R. Jr. et al. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 122, 537–557 (2010).
Taubert, D. et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb. Haemost. 92, 311–316 (2004).
Bouman, H.J. et al. Which platelet function test is suitable to monitor clopidogrel responsiveness? A pharmacokinetic analysis on the active metabolite of clopidogrel. J. Thromb. Haemost. 8, 482–488 (2010).
Pereillo, J.M. et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab. Dispos. 30, 1288–1295 (2002).
Dansette, P.M., Libraire, J., Bertho, G. & Mansuy, D. Metabolic oxidative cleavage of thioesters: evidence for the formation of sulfenic acid intermediates in the bioactivation of the antithrombotic prodrugs ticlopidine and clopidogrel. Chem. Res. Toxicol. 22, 369–373 (2009).
Kazui, M. et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 38, 92–99 (2010).
Wheeler, J.G., Keavney, B.D., Watkins, H., Collins, R. & Danesh, J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet 363, 689–695 (2004).
Billecke, S. et al. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 28, 1335–1342 (2000).
Adkins, S., Gan, K.N., Mody, M. & La Du, B.N. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am. J. Hum. Genet. 52, 598–608 (1993).
Harel, M. et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 11, 412–419 (2004).
Prentice, R.L. On the design of synthetic case-control studies. Biometrics 42, 301–310 (1986).
King, S.B. III et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation 117, 261–295 (2008).
Simon, T. et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009).
Angiolillo, D.J. et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler. Thromb. Vasc. Biol. 26, 1895–1900 (2006).
Suh, J.W. et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ 174, 1715–1722 (2006).
Brandt, J.T. et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436 (2007).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Gurbel, P.A., Becker, R.C., Mann, K.G., Steinhubl, S.R. & Michelson, A.D. Platelet function monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 50, 1822–1834 (2007).
Sibbing, D. et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 53, 849–856 (2009).
Clarke, T.A. & Waskell, L.A. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab. Dispos. 31, 53–59 (2003).
Correia, M.A. & Ortiz de Montellano, P.R. Inhibition of cytochrome P450 enzymes. in Cytochrome P450: Structure, Mechanism, and Biochemistry (ed. Ortiz de Montellano, P.R.) 253 (Kluwer Academic/Plenum Publishers, New York, 2005).
Kosaka, T., Yamaguchi, M., Motomura, T. & Mizuno, K. Investigation of the relationship between atherosclerosis and paraoxonase or homocysteine thiolactonase activity in patients with type 2 diabetes mellitus using a commercially available assay. Clin. Chim. Acta 359, 156–162 (2005).
Perla-Kaján, J. & Jakubowski, H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J. 24, 931–936 (2010).
Bertrand-Thiébault, C. et al. Genetic polymorphism of CYP2C19 gene in the Stanislas cohort. A link with inflammation. Ann. Hum. Genet. 72, 178–183 (2008).
Bhattacharyya, T. et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. J. Am. Med. Assoc. 299, 1265–1276 (2008).
Rainwater, D.L. et al. Determinants of variation in human serum paraoxonase activity. Heredity 102, 147–154 (2009).
Barlow, W.E., Ichikawa, L., Rosner, D. & Izumi, S. Analysis of case-cohort designs. J. Clin. Epidemiol. 52, 1165–1172 (1999).
Acknowledgements
We are grateful to all subjects who participated in the study. We thank numerous unnamed staff of our institutions for their efforts in subject recruitment, clinical, laboratory and experimental assessments.
Author information
Authors and Affiliations
Contributions
J.W.v.W. and D.T. conceived the project. E.S., J.M.t.B. and D.T. supervised the project. H.J.B., J.W.v.W., J.V., C.M.H., C.H., C.W., H.-G.S. and D.T. conducted or directed bioanalytics of clopidogrel and its metabolites, metabolomic profiling, blood collection and sample preparation, aggregometry, genotyping and PON1 phenotyping. H.J.B., E.S., J.W.v.W., C.M.H., J.M.t.B. and D.T. conducted or directed recruitment of subjects, disease assessment and follow-up assessments. H.J.B., J.W.v.W. and D.T. did the computational and statistical data analyses. H.J.B. and D.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–10, Supplementary Figures 1–9 and Supplementary Methods (PDF 589 kb)
Rights and permissions
About this article
Cite this article
Bouman, H., Schömig, E., van Werkum, J. et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17, 110–116 (2011). https://doi.org/10.1038/nm.2281
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2281
This article is cited by
-
Pleiotropic effects of clopidogrel
Purinergic Signalling (2022)
-
The disappearance of IPO in myocardium of diabetes mellitus rats is associated with the increase of succinate dehydrogenase-flavin protein
BMC Cardiovascular Disorders (2021)
-
Impact of genetic variants on major bleeding after percutaneous coronary intervention based on a prospective multicenter registry
Scientific Reports (2021)
-
New genetic variants associated with major adverse cardiovascular events in patients with acute coronary syndromes and treated with clopidogrel and aspirin
The Pharmacogenomics Journal (2021)
-
Pharmacogenetics to guide cardiovascular drug therapy
Nature Reviews Cardiology (2021)