Abstract
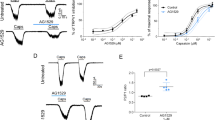
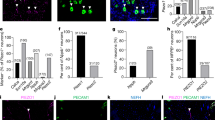
Topical application of nicotine, as used in nicotine replacement therapies, causes irritation of the mucosa and skin. This reaction has been attributed to activation of nicotinic acetylcholine receptors (nAChRs) in chemosensory neurons. In contrast with this view, we found that the chemosensory cation channel transient receptor potential A1 (TRPA1) is crucially involved in nicotine-induced irritation. We found that micromolar concentrations of nicotine activated heterologously expressed mouse and human TRPA1. Nicotine acted in a membrane-delimited manner, stabilizing the open state(s) and destabilizing the closed state(s) of the channel. In the presence of the general nAChR blocker hexamethonium, nociceptive neurons showed nicotine-induced responses that were strongly reduced in TRPA1-deficient mice. Finally, TRPA1 mediated the mouse airway constriction reflex to nasal instillation of nicotine. The identification of TRPA1 as a nicotine target suggests that existing models of nicotine-induced irritation should be revised and may facilitate the development of smoking cessation therapies with less adverse effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hatsukami, D.K., Stead, L.F. & Gupta, P.C. Tobacco addiction. Lancet 371, 2027–2038 (2008).
Thuerauf, N. et al. The influence of mecamylamine on trigeminal and olfactory chemoreception of nicotine. Neuropsychopharmacology 31, 450–461 (2006).
Stead, L.F., Perera, R., Bullen, C., Mant, D. & Lancaster, T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. CD000146 (2008).
Nides, M. Update on pharmacologic options for smoking cessation treatment. Am. J. Med. 121, S20–S31 (2008).
Hajek, P. et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray and an inhaler. Arch. Intern. Med. 159, 2033–2038 (1999).
Dussor, G.O. et al. Potentiation of evoked calcitonin gene–related peptide release from oral mucosa: a potential basis for the pro-inflammatory effects of nicotine. Eur. J. Neurosci. 18, 2515–2526 (2003).
Simons, C.T., Sudo, S., Sudo, M. & Carstens, E. Mecamylamine reduces nicotine cross-desensitization of trigeminal caudalis neuronal responses to oral chemical irritation. Brain Res. 991, 249–253 (2003).
Lee, L.Y. & Gu, Q. Cough sensors. IV. Nicotinic membrane receptors on cough sensors. Handb. Exp. Pharmacol. 187, 77–98 (2009).
Talavera, K., Nilius, B. & Voets, T. Neuronal TRP channels: thermometers, pathfinders and lifesavers. Trends Neurosci. 31, 287–295 (2008).
Damann, N., Voets, T. & Nilius, B. TRPs in our senses. Curr. Biol. 18, R880–R889 (2008).
Venkatachalam, K. & Montell, C. TRP channels. Annu. Rev. Biochem. 76, 387–417 (2007).
Liu, L. et al. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J. Neurophysiol. 91, 1482–1491 (2004).
Fucile, S., Sucapane, A. & Eusebi, F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J. Physiol. (Lond.) 565, 219–228 (2005).
Bessac, B.F. & Jordt, S.E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370 (2008).
Story, G.M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Karashima, Y. et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 106, 1273–1278 (2009).
Jordt, S.E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Andrè, E. et al. Cigarette smoke–induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 (2008).
Bautista, D.M. et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006).
Kwan, K.Y. et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 (2006).
MacPherson, L.J. et al. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 32, 335–343 (2006).
Karashima, Y. et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 27, 9874–9884 (2007).
Xiao, B. et al. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 28, 9640–9651 (2008).
Meseguer, V. et al. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J. Neurosci. 28, 576–586 (2008).
Fajardo, O., Meseguer, V., Belmonte, C. & Viana, F. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin) 2, 429–438 (2008).
Hinman, A., Chuang, H.H., Bautista, D.M. & Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 103, 19564–19568 (2006).
Macpherson, L.J. et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 (2007).
Zurborg, S., Yurgionas, B., Jira, J.A., Caspani, O. & Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 10, 277–279 (2007).
Doerner, J.F., Gisselmann, G., Hatt, H. & Wetzel, C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 282, 13180–13189 (2007).
Carstens, E., Kuenzler, N. & Handwerker, H.O. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol. 80, 465–492 (1998).
Simons, C.T., Boucher, Y., Carstens, M.I. & Carstens, E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J. Neurophysiol. 96, 1877–1886 (2006).
Papke, R.L., Sanberg, P.R. & Shytle, R.D. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 297, 646–656 (2001).
Xu, H., Blair, N.T. & Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 25, 8924–8937 (2005).
Liu, L. & Simon, S.A. Capsaicin and nicotine both activate a subset of rat trigeminal ganglion neurons. Am. J. Physiol. 270, C1807–C1814 (1996).
Haberberger, R.V. et al. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton. Neurosci. 113, 32–42 (2004).
Widdicombe, J. Reflexes from the lungs and airways: historical perspective. J. Appl. Physiol. 101, 628–634 (2006).
Vanoirbeek, J.A. et al. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol. Sci. 80, 310–321 (2004).
Kreslake, J.M., Wayne, G.F. & Connolly, G.N. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob. Res. 10, 705–715 (2008).
Dessirier, J.M., O'Mahony, M. & Carstens, E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol. Behav. 73, 25–36 (2001).
Zanotto, K.L., Merrill, A.W., Carstens, M.I. & Carstens, E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol and other irritant stimuli. J. Neurophysiol. 97, 966–978 (2007).
Chung, M.K., Lee, H., Mizuno, A., Suzuki, M. & Caterina, M.J. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 24, 5177–5182 (2004).
Dhar, P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 35, 155–168 (2004).
Carstens, E., Albin, K.C., Simons, C.T. & Carstens, M.I. Time course of self-desensitization of oral irritation by nicotine and capsaicin. Chem. Senses 32, 811–816 (2007).
Togias, A. Mechanisms of nose-lung interaction. Allergy 54 Suppl 57: 94–105 (1999).
Dhaka, A. et al. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 (2007).
Rask, L. et al. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 42, 93–113 (2000).
Baldwin, I. & Ohnmeiss, T. Alkaloidal responses to damage in Nicotiana native to North America. J. Chem. Ecol. 19, 1143–1153 (1993).
Talavera, K., Janssens, A., Klugbauer, N., Droogmans, G. & Nilius, B. Extracellular Ca2+ modulates the effects of protons on gating and conduction properties of the T-type Ca2+ channel α1G (CaV3.1). J. Gen. Physiol. 121, 511–528 (2003).
Southam, D.S., Dolovich, M., O'Byrne, P.M. & Inman, M.D. Distribution of intranasal instillations in mice: effects of volume, time, body position and anesthesia. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L833–L839 (2002).
Acknowledgements
We are grateful to K.Y. Kwan for providing us with the Trpa1 knockout mice, M.R. Sepúlveda for helpful discussions, and V. De Vooght and P. Hoet for help in some plethysmography experiments. The expert technical assistance of J. Prenen is greatly acknowledged. The mTRPA1 CHO cell line was kindly provided by A. Patapoutian. K.T. and J.A.J.V. were supported by a postdoctoral mandate from KU Leuven and are currently postdoctoral fellows of the Research Foundation–Flanders (Fonds voor Wetenschappelijk Onderzoek, FWO). M.G. and W.E. are doctoral FWO fellows. V.M.M was supported by Spanish CONSOLIDER-INGENIO 2010 CSD2007-00023. This work was supported by grants from Inter-university Attraction Poles Programme (Belgian Science Policy, P6/28), FWO (G.0172.03 and G.0565.07), the Research Council of the KU Leuven (GOA 2004/07) and the Flemish Government (Excellentiefinanciering, EF/95/010).
Author information
Authors and Affiliations
Contributions
K.T. carried out patch-clamp and Ca2+-imaging recordings, plethysmography experiments, analyzed the data, wrote the paper and supervised the project. M.G. and Y.K. performed patch-clamp and Ca2+-imaging recordings. V.M.M. carried out patch-clamp and Ca2+-imaging recordings in neurons. J.A.J.V. performed plethysmography experiments. N.D. carried out Ca2+-imaging recordings in neurons and edited the paper. W.E. performed Ca2+-imaging and mouse experiments and edited the paper. M.B. carried out mouse genotyping. A.J. performed the molecular biology work. R.V. supervised mouse genotyping and edited the paper. F.V. edited the paper and supervised the project. B. Nemery edited the paper and supervised the plethysmography experiments. B. Nilius edited the paper and supervised the project. T.V. analyzed the data, wrote the paper and supervised the project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 3128 kb)
Rights and permissions
About this article
Cite this article
Talavera, K., Gees, M., Karashima, Y. et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12, 1293–1299 (2009). https://doi.org/10.1038/nn.2379
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2379
This article is cited by
-
The Effect of Anti-Chemokine Oral Drug XC8 on Cough Triggered by The Agonists of TRPA1 But Not TRPV1 Channels in Guinea Pigs
Pulmonary Therapy (2022)
-
Acute cigarette smoke or extract exposure rapidly activates TRPA1-mediated calcium influx in primary human airway smooth muscle cells
Scientific Reports (2021)
-
TRPA1 gene variants hurting our feelings
Pflügers Archiv - European Journal of Physiology (2020)
-
To flourish or perish: evolutionary TRiPs into the sensory biology of plant-herbivore interactions
Pflügers Archiv - European Journal of Physiology (2019)
-
Differential interactions of bacterial lipopolysaccharides with lipid membranes: implications for TRPA1-mediated chemosensation
Scientific Reports (2018)