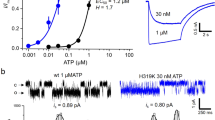
Abstract
In the closed structure of the P2X cation channel, three α-helical transmembrane domains cross the membrane obliquely. In rat P2X2 receptors, these intersect at Thr339. Replacing Thr339 by lysine in one, two or three subunits progressively increased chloride permeability and reduced unitary conductance. This implies that the closed-open transition involves a symmetrical separation of the three subunits and that Thr339 from each subunit contributes symmetrically to the open channel permeation pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
North, R.A. Physiol. Rev. 82, 1013–1067 (2002).
Kawate, T., Michel, J.C., Birdsong, W.T. & Gouaux, E. Nature 460, 592–598 (2009).
Egan, T.M., Haines, W.R. & Voigt, M.M. J. Neurosci. 18, 2350–2359 (1998).
Li, M., Chang, T.H., Silberberg, S.D. & Swartz, K.J. Nat. Neurosci. 11, 883–887 (2008).
Rassendren, F., Buell, G., Newbolt, A., North, R.A. & Surprenant, A. EMBO J. 16, 3446–3454 (1997).
Migita, K., Haines, W.R., Voigt, M.M. & Egan, T.M. J. Biol. Chem. 276, 30934–30941 (2001).
Cao, L., Broomhead, H.E., Young, M.T. & North, R.A. J. Neurosci. 29, 14257–14264 (2009).
Cao, L., Young, M.T., Broomhead, H.E., Fountain, S.J. & North, R.A. J. Neurosci. 27, 12916–12923 (2007).
Zhou, Z. & Hume, R.I. J. Physiol. (Lond.) 507, 353–364 (1998).
Bo, X. et al. Mol. Pharmacol. 63, 1407–1416 (2003).
Galzi, J.L. et al. Nature 359, 500–505 (1992).
Qu, W. et al. J. Gen. Physiol. 127, 375–389 (2006).
Silberberg, S.D., Li, M. & Swartz, K.J. Neuron 54, 263–274 (2007).
Browne, L.E., Jiang, L.H. & North, R.A. Trends Pharmacol. Sci. 31, 229–237 (2010).
Kracun, S., Chaptal, V., Abramson, J. & Khakh, B.S. J. Biol. Chem. 285, 10110–10121 (2010).
Acknowledgements
This work was supported by the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
R.A.N., L.E.B. and L.C. conceived and designed the experiments and analyzed the data. H.E.B., L.B. and W.J.W. generated the constructs and carried out western blotting. L.E.B. and L.C. performed the single-channel and whole-cell electrophysiology. L.E.B. constructed molecular models. R.A.N. wrote the paper with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Table 1 and Supplementary Methods (PDF 1011 kb)
Rights and permissions
About this article
Cite this article
Browne, L., Cao, L., Broomhead, H. et al. P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat Neurosci 14, 17–18 (2011). https://doi.org/10.1038/nn.2705
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2705
This article is cited by
-
Enlightening activation gating in P2X receptors
Purinergic Signalling (2022)
-
Unravelling the intricate cooperativity of subunit gating in P2X2 ion channels
Scientific Reports (2020)
-
Insights into the channel gating of P2X receptors from structures, dynamics and small molecules
Acta Pharmacologica Sinica (2016)
-
Molecular mechanism of ATP binding and ion channel activation in P2X receptors
Nature (2012)
-
Tightening of the ATP-binding sites induces the opening of P2X receptor channels
The EMBO Journal (2012)