Abstract
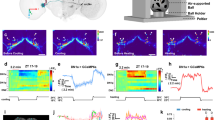
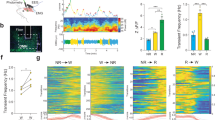
How animals maintain proper amounts of sleep yet remain flexible to changes in environmental conditions remains unknown. We found that environmental light suppressed the wake-promoting effects of dopamine in fly brains. The ten large lateral-ventral neurons (l-LNvs), a subset of clock neurons, are wake-promoting and respond to dopamine, octopamine and light. Behavioral and imaging analyses suggested that dopamine is a stronger arousal signal than octopamine. Notably, light exposure not only suppressed l-LNv responses, but also synchronized responses of neighboring l-LNvs. This regulation occurred by distinct mechanisms: light-mediated suppression of octopamine responses was regulated by the circadian clock, whereas light regulation of dopamine responses occurred by upregulation of inhibitory dopamine receptors. Plasticity therefore alters the relative importance of diverse cues on the basis of the environmental mix of stimuli. The regulatory mechanisms described here may contribute to the control of sleep stability while still allowing behavioral flexibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ganguly-Fitzgerald, I., Donlea, J. & Shaw, P.J. Waking experience affects sleep need in Drosophila. Science 313, 1775–1781 (2006).
Ho, K.S. & Sehgal, A. Drosophila melanogaster: an insect model for fundamental studies of sleep. Methods Enzymol. 393, 772–793 (2005).
Keene, A.C. et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 20, 1209–1215 (2010).
Shang, Y., Griffith, L.C. & Rosbash, M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. USA 105, 19587–19594 (2008).
Andretic, R., van Swinderen, B. & Greenspan, R.J. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15, 1165–1175 (2005).
Crocker, A. & Sehgal, A. Octopamine regulates sleep in Drosophila through protein kinase A–dependent mechanisms. J. Neurosci. 28, 9377–9385 (2008).
Crocker, A., Shahidullah, M., Levitan, I.B. & Sehgal, A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65, 670–681 (2010).
Parisky, K.M. et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60, 672–682 (2008).
Hendricks, J.C. et al. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000).
Shaw, P.J., Cirelli, C., Greenspan, R.J. & Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000).
Sheeba, V. et al. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 18, 1537–1545 (2008).
Donlea, J.M., Ramanan, N. & Shaw, P.J. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 324, 105–108 (2009).
Sheeba, V., Gu, H., Sharma, V.K., O'Dowd, D.K. & Holmes, T.C. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J. Neurophysiol. 99, 976–988 (2008).
Renn, S.C., Park, J.H., Rosbash, M., Hall, J.C. & Taghert, P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802 (1999).
Shafer, O.T. et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58, 223–237 (2008).
Kula-Eversole, E. et al. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA 107, 13497–13502 (2010).
Shakiryanova, D. & Levitan, E.S. Prolonged presynaptic posttetanic cyclic GMP signaling in Drosophila motoneurons. Proc. Natl. Acad. Sci. USA 105, 13610–13613 (2008).
Feinberg, E.H. et al. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, 353–363 (2008).
Gordon, M.D. & Scott, K. Motor control in a Drosophila taste circuit. Neuron 61, 373–384 (2009).
Crocker, A. & Sehgal, A. Genetic analysis of sleep. Genes Dev. 24, 1220–1235 (2010).
Hamada, F.N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008).
Chiang, A.S. et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 21, 1–11 (2011).
Mao, Z. & Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural. Circuits 3, 5 (2009).
Sugamori, K.S., Demchyshyn, L.L., McConkey, F., Forte, M.A. & Niznik, H.B. A primordial dopamine D1-like adenylyl cyclase–linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 362, 131–138 (1995).
Hall, J.C. Systems approaches to biological rhythms in Drosophila. Methods Enzymol. 393, 61–185 (2005).
Bonci, A. & Hopf, F.W. The dopamine D2 receptor: new surprises from an old friend. Neuron 47, 335–338 (2005).
Han, K.A., Millar, N.S., Grotewiel, M.S. & Davis, R.L. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron 16, 1127–1135 (1996).
Hearn, M.G. et al. A Drosophila dopamine 2–like receptor: molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. USA 99, 14554–14559 (2002).
Nitabach, M.N., Sheeba, V., Vera, D.A., Blau, J. & Holmes, T.C. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J. Neurobiol. 62, 1–13 (2005).
Wijnen, H., Naef, F., Boothroyd, C., Claridge-Chang, A. & Young, M.W. Control of daily transcript oscillations in Drosophila by light and the circadian clock. PLoS Genet. 2, e39 (2006).
Helfrich-Förster, C. et al. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J. Comp. Neurol. 500, 47–70 (2007).
Wang, J.W., Wong, A.M., Flores, J., Vosshall, L.B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003).
Cayre, M., Buckingham, S.D., Yagodin, S. & Sattelle, D.B. Cultured insect mushroom body neurons express functional receptors for acetylcholine, GABA, glutamate, octopamine and dopamine. J. Neurophysiol. 81, 1–14 (1999).
Gervasi, N., Tchenio, P. & Preat, T. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron 65, 516–529 (2010).
Soille, P. Morphological Image Analysis: Principles and Applications (Springer-Verlag, Secaucus, New Jersey, USA, 2003).
Meyer, F. Topographic distance and watershed lines. Signal Processing 38, 113–125 (1994).
Acknowledgements
We thank P. Taghert for kindly providing pdfGal4 (X) and UAS-EPAC flies. We obtained UAS-D2R-RNAi lines from the Vienna Drosophila RNAi center. We are grateful to O. Shafer for technical help with cAMP imaging. We also thank E. Dougherty for assistance in confocal microscopy, K. Palm and S. Pescatore for administrative assistance and C. Vecsey for comments on the manuscript. The work was supported in part by grants from the US National Institutes of Health (PO1 NS044232-06 to M.R., R01 MH067284 to L.C.G. and NIH R01 EB007042 to P. Hong).
Author information
Authors and Affiliations
Contributions
Y.S. conceived the project. Y.S., P. Haynes, N.P. and F.G. performed the experiments. K.I.H., J.P. and P. Hong developed the algorithm for the automated imaging analysis. Y.S., L.C.G. and M.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 454 kb)
Rights and permissions
About this article
Cite this article
Shang, Y., Haynes, P., Pírez, N. et al. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 14, 889–895 (2011). https://doi.org/10.1038/nn.2860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2860
This article is cited by
-
A four-oscillator model of seasonally adapted morning and evening activities in Drosophila melanogaster
Journal of Comparative Physiology A (2023)
-
The Role of the Dopamine Transporter in the Effects of Amphetamine on Sleep and Sleep Architecture in Drosophila
Neurochemical Research (2022)
-
Compartment specific regulation of sleep by mushroom body requires GABA and dopaminergic signaling
Scientific Reports (2021)
-
Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive
Neuropsychopharmacology (2018)
-
Aberrant Axonal Arborization of PDF Neurons Induced by Aβ42-Mediated JNK Activation Underlies Sleep Disturbance in an Alzheimer’s Model
Molecular Neurobiology (2017)