Abstract
The muscarinic cholinergic receptor (mAChR) antagonist scopolamine was used to induce transient cognitive impairment in monkeys trained in a delayed matching to sample task. The temporal relationship between the occupancy level of central mAChRs and cognitive impairment was determined. Three conscious monkeys (Macaca mulatta) were subjected to positron emission tomography (PET) scans with the mAChR radioligand N-[11C]methyl-3-piperidyl benzilate ([11C](+)3-MPB). The scan sequence was pre-, 2, 6, 24, and 48 h post-intramuscular administration of scopolamine in doses of 0.01 and 0.03 mg/kg. Occupancy levels of mAChR were maximal 2 h post-scopolamine in cortical regions innervated primarily by the basal forebrain, thalamus, and brainstem, showing that mAChR occupancy levels were 43–59 and 65–89% in doses of 0.01 and 0.03 mg/kg, respectively. In addition, dose-dependent impairment of working memory performance was measured 2 h after scopolamine. A positive correlation between the mAChR occupancy and cognitive impairment 2 and 6 h post-scopolamine was the greatest in the brainstem (P<0.00001). Although cognitive impairment was not observed 24 h post-scopolamine, sustained mAChR occupancy (11–24%) was found with both doses in the basal forebrain and thalamus, but not in the brainstem. These results indicate that a significant degree of mAChRs occupancy is needed to produce cognitive impairment by scopolamine. Furthermore, the importance of the brainstem cholinergic system in working memory in monkey is described.
Similar content being viewed by others
INTRODUCTION
Muscarinic cholinergic receptor (mAChR) neurotransmission in the central nervous system is involved in human cognitive function including attention and working memory (Collerton, 1986; Everitt and Robbins, 1997; Hasselmo and Stern, 2006; Kobayashi et al, 2002; Sellin et al, 2008). Scopolamine, a non-selective mAChR antagonist, induces transient cognitive impairment (Broks et al, 1988; Drachman, 1977; Edginton and Rusted, 2003; Green et al, 2005; Potter et al, 2000; Rasmusson and Dudar, 1979; Robbins et al, 1997; Rusted and Warburton, 1988). Acetylcholinesterase inhibitors such as physostigmine reverse the cognitive impairment induced by scopolamine in human beings (Honer et al, 1988; Mewaldt and Ghoneim, 1979; Prohovnik et al, 1997; Sitaram et al, 1978). Animal studies have also shown that scopolamine induces transient cognitive impairment in a dose-dependent manner (Aigner and Mishkin, 1986; Bartus and Johnson, 1976; Hardman and Limbird, 1996; Hudzik and Wenger, 1993; Plakke et al, 2008; Spinelli et al, 2006; Taffe et al, 1999, 2002).
Cholinergic projection neurons are located in two main regions in the brain (Mesulam et al, 1983, 1984). One is in the basal forebrain, which projects mainly to the hippocampus and most of the neocortex (Mesulam et al, 1983, 1986), and the other is in the brainstem, which innervates the thalamus as well as the basal forebrain (Mesulam et al, 1983). Although much attention and memory research has focused on the basal forebrain cholinergic systems (Collerton, 1986; Hasselmo and Stern, 2006; Sellin et al, 2008), a significant role of the brainstem cholinergic system has been reported on attention, learning, and memory (Kobayashi et al, 2002; Kozak et al, 2005; Mitchell et al, 2002; Rostron et al, 2008).
Abnormalities of the central mAChR system in Alzheimer disease are well correlated with the degree of dementia (Höhmann et al, 1998; Perry, 1986; Terry and Buccafusco, 2003). Reductions in central mAChR systems are present not only in Alzheimer-type dementia (Rinne et al, 1985; Reinikainen et al, 1987), but also in Huntington's disease (Enna et al, 1976; Lange et al, 1992; Wastek and Yamamura, 1978), Parkinson's disease (Ahlskog et al, 1991), and schizophrenia (Crook et al, 2000; Dean et al, 2002; Zavitsanou et al, 2004). Several mAChR agonists improve cognitive function in Alzheimer-type dementia (Bodick et al, 1997; Caccamo et al, 2009; Langmead et al, 2008) and schizophrenia (Raedler et al, 2003; Sellin et al, 2008; Shekhar et al, 2008).
The delayed matching to sample (DMS) task in monkeys is one method for evaluating potential drug effects on cognitive functions (Bartus and Johnson, 1976; Hampson et al, 2009; Penetar and McDonough, 1983). Typically, a sample visual stimulus is presented to the animal for a short time. Following a delay interval, the sample and another test stimulus are presented simultaneously. The subject is required to choose the sample visual stimulus to be rewarded. One limitation of conventional DMS task is that the maximal delay interval is fixed for all subjects, although cognitive abilities differ among them. Delay interval variation based on the cognitive ability of each subject is especially suitable for detection of drug effects (Buccafusco et al, 1995). In the titration version of the DMS (T-DMS) task, the delay interval varies depending on subject's on-going performance. Thus, when responding correctly, the delay interval becomes longer, and when the responding in error, the delay interval becomes shorter (Buccafusco et al, 2002, 2003; Hudzik and Wenger, 1993).
In this study, the temporal relationship between the occupancy level of central mAChRs and the degree of cognitive impairment induced by scopolamine was assessed in monkeys (Macaca mulatta). The occupancy of mAChRs was measured with N-[11C]methyl-3-piperidyl benzilate ([11C](+)3-MPB) using a high-resolution animal positron emission tomography (PET; Tsukada et al, 2001b, 2001c) during the conscious awake state. The degree of cognitive impairment was determined by the T-DMS task described above.
MATERIALS AND METHODS
Subjects and Design
Three male rhesus monkeys (M. mulatta; 5–6 years old), weighing 4–5 kg, were studied. Experiments were conducted in accordance with the recommendations of the US National Institute of Health and the guidelines of the Central Research Laboratory, Hamamatsu Photonics (Hamamatsu, Japan). Scopolamine hydrobromide was obtained from Kyorin Pharmaceutical (Tokyo, Japan). The doses of scopolamine salt (0.01 and 0.03 mg/kg, intramuscularly in 0.1 ml/kg) were similar to previous monkey studies (Aigner and Mishkin, 1986; Bartus and Johnson, 1976; Hudzik and Wenger, 1993; Spinelli et al, 2006).
First, cognitive functions were assessed using the T-DMS task pre-, 2, 6, 24, and 48 h post-saline vehicle or scopolamine in intramuscular doses of 0.01 and 0.03 mg/kg. The T-DMS task of vehicle or scopolamine at each dose was administered twice with a 1-week interval, respectively. The order of vehicle and scopolamine at each dose was counterbalanced across subjects. Next, the time course of mAChR occupancy was investigated by PET measurements with [11C](+)3-MPB in same conscious animals. Consecutive PET measurements were performed pre-, 2, 6, 24, and 48 h post-scopolamine at each dose, respectively. Thirty PET measurements were performed in the three monkeys. Each monkey was examined 10 times at five time points (pre-, 2, 6, 24, and 48 h post-scopolamine), with both doses of 0.01 and 0.03 mg/kg. Four sessions were performed during each time point pre- and post-vehicle or scopolamine. Scopolamine was administered a total of six times (the T-DMS task four times, and twice for PET measurement) for each monkey.
Behavioral Testing
The procedure of T-DMS task was based on previous monkey studies by Buccafusco et al (2002, 2003). Sixty-four different visual stimuli comprising all combinations of eight distinct colors and eight distinct shapes were used. Each visual stimulus was presented on a black background on a touch-sensitive screen placed within the animals’ reach. The size of each stimulus was 6 cm2. Sessions consisted of 32 trials arranged so that each shape was presented as a sample stimulus four times. In one trial, colors of the sample and test stimuli were same. Each trial began with presentation of a start cue (a white circle) on the lower side of the touch-sensitive screen. The animal had to press a circular button within 5 s after the onset of the start cue. Pressing the button changed the start cue to a red-fixation point. If the monkey pushed the button for 1 s, the red-fixation point disappeared and a sample stimulus appeared for 300 ms at the location of the start cue. After the delay interval, a sample stimulus and three other stimuli appeared in an arch-like arrangement. Each monkey had to touch the visual stimulus, which matched the sample within 5 s. Water (0.2 ml) was given as a reward for a correct choice. In the first trial, a sample stimulus at the location of the start cue did not disappear, and the delay interval was not introduced. If the trial was correct, the next trial was presented with a delay interval 1 s longer. This progression was maintained until the monkey responded incorrectly. Incorrect choices resulted in an error signal in which all items disappeared accompanied by 5 s beep sound (100 Hz) and visual presentation of a large purple rectangle. The delay interval for the trial after an incorrect choice was decreased by 1 s. The reaction time (RT) from the test stimulus presentation on the screen to monkey's first touch of it was measured. Averaging the delay interval over the last 10 trials of each session was used as the cognitive index. Monkeys were trained for eight sessions (1 session=32 trials) per day. Each session took 7–10 min, and the interval between sessions was 3 min. Monkeys were well trained for about 1 year before any drug use. Cognitive impairment was defined as follows:

where Cognitive Indexpre and Cognitive Indexpost are pre- and post-administration of vehicle or scopolamine, respectively.
Synthesis of [11C](+)3-MPB
Carbon-11 (11C) was produced by the 14N(p,α)11C nuclear reaction using a cyclotron (HM-18, Sumitomo Heavy Industry, Tokyo, Japan) at Hamamatsu Photonics PET Center obtained as [11C]CO2. [11C](+)3-MPB was labeled by N-methylation of the nor-compound with [11C]methyl iodide (Nishiyama et al, 2001; Takahashi et al, 1999; Tsukada et al, 2001b, 2001c). The radioactive purity was greater than 99%. Specific radioactivity was 83.0±25.6 GBq/μmol (mean±SD) and mass injected was 4.4±1.9 μg (mean±SD). After analysis, the solution was passed through a 0.22 μm pore filter before intravenous administration.
PET Scans
A high-resolution animal PET scanner (SHR-7700; Hamamatsu Photonics) with a transaxial resolution of 2.6 mm full-width at half-maximum in the enhanced two-dimensional mode and a center-to-center distance of 3.6 mm (Watanabe et al, 1997) was used. To avoid excessive arterial blood sampling, PET scans were performed without sampling. For quantitative analysis, the binding potential non-displaceable (BPND) was determined by Logan plots, with the cerebellum as the reference region (Yamamoto et al, 2011).
A saphenous venous cannula was inserted in an inferior limb. The animal's head was rigidly fixed to the upper frame of a monkey chair using an acrylic head restraining device. The animal sitting in the restraining chair was placed at a fixed position in the PET gantry, with stereotactic coordinates aligned parallel to the orbito-meatal plane. Transmission data with a 68Ge–68Ga pin source was obtained for attenuation correction. After intravenous bolus injection of [11C](+)3-MPB (200 MBq/kg body weight), PET scans were acquired for 91 min. The injected dose of each animal was 270.5±28.9, 299.1±31.2, and 263.3±18.5 MBq/kg (mean±SD), respectively. A summation image from 28 to 40 min post-injection was obtained. The brain MRI was co-registered to the PET image.
Working memory has been reported to involve multiple brain regions such as the dorsolateral prefrontal cortex (Funahashi et al, 1989; Owen, 2000; Sawaguchi and Goldman-Rakic, 1991), inferior temporal cortex (Tang et al, 1997; Turchi et al, 2005), parietal cortex (Chafee and Goldman-Rakic, 1998), occipital cortex (Supèr et al, 2001), hippocampus (Hannula et al, 2006), thalamus (Mitchell et al, 2002), brainstem (Inglis et al, 2001), and striatum (Kitabatake et al, 2003). Therefore, the binding of [11C](+)3-MPB in these eight brain regions was investigated. The above brain regions of interest (ROIs) were obtained based on registered MRI using the cerebellum as a reference.
Graphic Analysis
For quantitative analysis, the Logan reference tissue method was performed in pixel-wise kinetic modeling (PMOD) software (PMOD Group, Zurich, Switzerland). This method has been already validated in our previous study (Yamamoto et al, 2011). The Logan reference tissue method allows the estimation of distribution volume ratio (DVR), which can be expressed as follows (Logan et al, 1990, 1996):

where ROItar and ROIref are the radioactivity concentrations of the target and reference region, respectively, at time T. The DVR is the slope and k2 is the clearance rate from the reference region. A k2 value of 0.31 was used, according to a previous study (Yamamoto et al, 2011). C is the intercept of Y axis. DVR is the ratio of the distribution volume in the target to reference region. DVR minus one was calculated as BPND, which is the ratio at equilibrium of specifically bound radioligand to that of non-displaceable radioligand in the tissue (Innis et al, 2007). Data recorded during the first 15 min were excluded based on our previous PET study (Tsukada et al, 2001c). Voxel-wise maps of the BPND were calculated using the PMOD software.
Occupancy Estimation
Occupancy levels were determined from the degree of reduction (%) of the BPND by scopolamine. The mAChR occupancy was estimated as:

where BPND pre(ROI) and BPND post(ROI) are BPND pre- and post-scopolamine, respectively.
Statistics
Data were expressed as mean±SD. Statistical analyses of these data were performed using two-way repeated measures ANOVA, followed by Bonferroni comparison. The level of significance was P<0.05.
RESULTS
Figure 1a illustrates a typical delay interval (s) plotted against the trial number from one session of the T-DMS task with pre- and 2 h post-scopolamine in doses of 0.01 and 0.03 mg/kg. At the control pre-drug status, the monkeys’ correct response depended on each animal's working memory capacity. The mean cognitive index±SD for control period in each animal was 21±2.4, 21±2.2, 18±2.2 s, respectively. Scopolamine decreased the maximal delay interval in a dose-dependent manner 2 h post-administration. The temporal changes in cognitive performance are shown in Figure 1b for all three animals. The saline vehicle had no effect on either the cognitive index or RT. Cognitive impairment was maximum 2 h post-scopolamine in both doses, in a dose-dependent manner. Cognitive impairment was observed up to 6 h post-administration, thus a trend for dose-dependency at 2 h in the degree of cognitive impairment. Cognitive function recovered to the pre-scopolamine condition 24 h later. The RT was not affected by scopolamine.
Effects of scopolamine on the titration-delayed match to sample (T-DMS) task. (a) Typical changes in the delay interval were plotted against the trial number in one session pre- and 2 h post-scopolamine in doses of 0.01 and 0.03 mg/kg. (b) Time course of the cognitive index and reaction time (RT) following vehicle or scopolamine. Open circles correspond to pre-scopolamine or post-vehicle. Closed squares and closed triangles correspond to scopolamine in doses of 0.01 and 0.03 mg/kg, respectively. Statistical differences were established using two-way repeated measures analysis of variance (ANOVA) (P<0.05) with dose and time post-scopolamine as factors. The Bonferroni test confirmed a significant difference at **P<0.01 compared with baseline at each dose and ##P<0.01 comparison at each time point. The data in (b) are mean±SD.
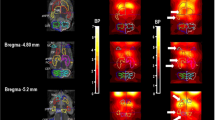
Representative maps of the BPND of [11C](+)3-MPB using the Logan plot compared with the reference region are presented in Figure 2. The BPND of [11C](+)3-MPB in the pre-scopolamine scan was the highest in the striatum, followed by cerebral cortices, hippocampus, thalamus, and lowest in the brainstem. The distribution pattern of [11C](+) 3-MPB is consistent with our previous PET studies (Nishiyama et al, 2001; Tsukada et al, 2001b, 2001c). Compared with the pre-scopolamine scan, the binding of [11C](+)3-MPB in all brain regions, except in the cerebellum, was decreased 2 and 6 h post-scopolamine in a dose-dependent manner. It completely recovered to pre-scopolamine levels 48 h post-administration. The mean voxel-wise estimates of BPND in the ROI in all scans were very similar to BPND using time–activity curves (TAC, y=0.98x+0.02, r=0.999, P<1.0 × 10−7).
Typical magnetic resonance imaging (MRI) and parametric positron emission tomography (PET) images of N-[11C]methyl-3-piperidyl benzilate ([11C](+)3-MPB) binding in monkey brain. For each group, five transverse slices referenced to the orbital meatal (OM) line plane at −3.6, +0, +14.4, +21.6, and +24.8 mm are shown. Each regions of interest (ROIs) of dorsolateral prefrontal cortex (light blue), inferior temporal cortex (green), parietal cortex (orange), occipital cortex (yellow), hippocampus (black), thalamus (red), brainstem (purple), striatum (blue), and cerebellum (white) were superimposed on the MRI image. Parametric maps of BPND of [11C](+)3-MPB were calculated by the Logan plot reference tissue method. The cerebellum was used as the reference region.
TACs of [11C](+)3-MPB pre- and post-scopolamine administration in seven ROIs and cerebellum are shown in Figure 3. The rank order of uptake of [11C](+)3-MPB was: striatum>cortices>thalamus, brainstem>cerebellum in all scans. In the pre-scopolamine scan, the TAC of the striatum showed the maximum accumulation around 50–60 min after injection. TACs of the cerebral cortices and hippocampus, thalamus and brainstem, and cerebellum showed a peak value around 30–40, 20–30, and 10 min after injection, respectively, followed by a gradual decrease with time. Inhibition of the uptake of [11C](+)3-MPB in these regions, except for the cerebellum 2 and 6 h post-scopolamine in doses of 0.01 and 0.03 mg/kg, was observed in a dose-dependent manner. Peak times of TAC in these seven regions shifted to an earlier period with scans 2 and 6 h post-scopolamine in a dose-dependent manner. Such effects were not observed in the cerebellum. The uptake of [11C](+)3-MPB in the striatum, cerebral cortices, hippocampus, and thalamus 24 h after scopolamine with both doses almost recovered to pre-scopolamine scan levels, and recovered to control by 48 h. The uptake of [11C](+)3-MPB in the brainstem recovered to pre-scopolamine levels by 24 h.
Effects of scopolamine on time–activity curves (TAC) of N-[11C]methyl-3-piperidyl benzilate ([11C](+)3-MPB) distribution in monkey brain. Typical TAC pre-, 2, 6, 24, and 48 post-scopolamine in doses of 0.01 and 0.03 mg/kg are shown. Regions of interests (ROIs) were identified according to the magnetic resonance imaging (MRI) of each monkey. The following regions are included: dorsolateral prefrontal cortex (•), parietal cortex (○), striatum (▪), hippocampus (□), inferior temporal cortex (▴), occipital cortex (♦), brainstem (◊), thalamus ( × ), and cerebellum (▵).
The temporal changes in the mAChR occupancy are shown in Figure 4. As the occupancy values were similar in basal forebrain innervated areas: dorsolateral prefrontal, inferior temporal, parietal, occipital cortices, and hippocampus, they were combined as cortices. BPND value and mAChR occupancy of each monkey are shown in Table 1. A dose-dependent occupancy was observed in all regions 2 h post-scopolamine. Occupancy was highest in the cortices. A dose-dependent occupancy was also observed 6 h post-scopolamine in the cortices, thalamus, and striatum, but not in the brainstem. Sustained ligand occupancy (11–24%) was still observed 24 h post-scopolamine in the cortices, thalamus, and striatum, but not in the brainstem. Occupancy in cortices, thalamus, and striatum was absent 48 h post-scopolamine.
Temporal changes in muscarinic cholinergic receptor (mAChR) occupancy following scopolamine administration. The brain regions are: pooled cortex (a), thalamus (b), brainstem (c), and striatum (d). Statistical differences were established using two-way repeated measures analysis of variance (ANOVA) (P<0.05) with dose and time post-scopolamine as factors. Bonferroni tests confirmed a significant difference at *P<0.05 and **P< 0.01 compared with post-48 h scan. Bonferroni tests also confirmed significant differences at each time point at #P<0.05 and ##P<0.01. Values are mean±SD.
The relationships between the mAChR occupancy and the degree of cognitive impairment in each region at doses of 0.01 and 0.03 mg/kg are shown in Figure 5. Both regression lines drawn by data points of 2 and 6 h (straight line), and 2, 6, 24, and 48 h (dashed line) showed that there were significantly positive correlations between each mAChR occupancy and cognitive impairment in all brain regions, and the highest correlation in the brainstem (Figure 5c). Slope of regression line drawn by the data points of 2 and 6 h (straight line) is steeper than that of 2, 6, 24, and 48 h (dashed line) in the pooled cortex, thalamus, and brainstem, respectively, but not in the striatum. Each graph has 24 data points, although it seems that pooled cortex (Figure 5a) has only 23 data points owing to two overlapping points ((x, y)=(69, 24)).
Relationship between the each muscarinic cholinergic receptor (mAChR) occupancy and cognitive impairment. The brain regions include: pooled cortex (a), thalamus (b), brainstem (c), and striatum (d) were assessed by using of data obtained from 2 and 6 h post-scopolamine administration in doses of 0.01 and 0.03 mg/kg. Regression line was drawn through the data points of 2 and 6 h (solid line), and 2, 6, 24, and 48 h (dashed line).
DISCUSSION
This study makes a significant contribution to the knowledge of the role of the muscarinic cholinergic system and cognition. The temporal relationship between the occupancy level of central mAChRs and cognitive impairment induced by scopolamine was assessed with PET measurements and the T-DMS task in conscious monkeys. Scopolamine occupied mAChRs dose-dependently 2 h post-administration. Cognitive function was significantly impaired in a dose-dependent manner at that time. Cognitive impairment with scopolamine persisted for 6 h and recovered completely to pre-scopolamine level 24 h later. However, some mAChR occupancy by scopolamine was still observed in most brain areas, except the brainstem, 24 h after its administration. The muscarinic cholinergic system in the brainstem is clearly involved with the maintenance of an alert cognitive state as shown in this study. Cognitive performance determined with the T-DMS task was almost similar levels among the three monkeys in the control condition, decreased by scopolamine, and mostly recovered 24 h post-scopolamine administration (Figure 1b). The behavioral data at pre- and 2 h post-scopolamine is consistent with a previous study (Hudzik and Wenger, 1993). One difference observed in this study is that scopolamine also decreased the simultaneous matching accuracy task (the delay interval=0 s). Hudzik and Wenger (1993) introduced a 3 s delay interval at the first trial, and performed a simultaneous matching task at another trial. Their simultaneous matching accuracy task corresponds to this first trial in the T-DMS task. Our monkeys did not miss the first trial even after scopolamine administration. In another previous study, the maximum delay interval in young adult monkey was 35–45 s (Buccafusco et al, 2002). Our monkeys’ maximum delay intervals were not so long. One possible reason might be that chance level of our study (25% with four choices) was lower than their study (50% with two choices). Although there are several slight differences among this and previous studies, the slightly modified T-DMS task was very suitable for evaluating the cognitive impairment induced by scopolamine.
Working memory is considered to be mediated by distinct neural circuits. Especially, significant roles of the thalamic-cortical-striatal circuit and hippocampus-cortical circuit have been suggested (Floresco et al, 1999). As described earlier, the thalamus, cerebral cortices, striatum, and hippocampus are innervated by cholinergic inputs (Mesulam et al, 1983; Warren et al, 2005). Local administration of scopolamine into the thalamus (Mitchell et al, 2002) or prefrontal cortex (Broersen et al, 1994; Herremans et al, 1996), or hippocampus (Ohno et al, 1992), and cholinergic cell ablation in the striatum (Kitabatake et al, 2003) produce impairment in working memory. Our present results are consistent with these previous findings. However, because the degree of cognitive impairment was most highly correlated with the mAChR in the brainstem, this structure becomes especially important. The brainstem mainly innervates the thalamus as well as the basal forebrain. It is one of the regions involved in motor control (arm movement: Matsumura et al (1997); eye movement: Dellu et al (1991); Kobayashi et al (2002)), attentional processing (Kobayashi et al, 2002; Kozak et al, 2005; Rostron et al, 2008), and motivational behavior (Bechara and van der Kooy, 1989; Stefurak and van der Kooy, 1994). Lesion of the brainstem cholinergic system induces deficits of attention (Inglis et al, 2001) or motivation (Steckler et al, 1994). Steckler et al (1994) suggested that deficits of motivation might affect on working memory indirectly. Perhaps, cognitive impairment induced by scopolamine is due to a motor, attention, and motivation deficit rather than working memory impairment. Over 80% occupancy of mAChRs in the striatum indicates its important role in the regulation of motor function (Alexander and Crutcher, 1990). These effects were observed 2 h post-administration in a dose of 0.03 mg/kg of scopolamine. However, the RTs post-scopolamine were not affected in this study (Figure 1b). This is reasonable because high levels of muscarinic blockade induce few motor deficits (Miller and Hiley, 1974). Blockade of mAChRs facilitates striatal DA neural signal transduction through DA1 and DA2 receptors (Tsukada et al, 2000, 2001a), and improves the motor impairments induced by dopamine receptor inhibition (Haraguchi et al, 1997). These data indicate that cognitive impairment was not due to a motor deficit. The animals in this study did not miss trials at short delay intervals even after scopolamine in a large dose of 0.03 mg/kg (Figure 1a). This suggests that the attentional function was not impaired and their motivation was not decreased. Cognitive impairment induced by scopolamine was not due to deficit of motor control, attention, and motivation, but to a working memory deficit. The brainstem cholinergic system must be considered as an important region involved in working memory.
As for subtypes of mAChRs (M1−5 receptors) have been identified by molecular cloning (Kubo et al, 1986), M1, M2, and M4 receptors are predominant subtypes expressed in different percentages among brain regions. Quantitative immunoprecipitation study indicates that the distribution percentage of M1, M2, and M4 receptor is ca 60, 20, and 20% in the cortex and hippocampus, respectively. In the striatum, the distribution percentage of M1, M2, and M4 receptor is ca 30, 20, and 50% (Flynn et al, 1995). The Ki values of (+)3-MPB for the human receptors from M1 to M5 were 1.34, 1.17, 2.82, 1.76, and 5.91 nM, respectively, as assessed with five cloned human mAChR subtypes expressed in CHO-K1 cells (unpublished data). Taken together with our result, the mAChR occupancy induced by scopolamine appears mainly to reflect M1 receptor occupancy in the cortices, and M4 receptor occupancy in the striatum, even though scopolamine is a non-selective mAChR antagonist. Therapeutic effects of M1/M4 receptor agonists on cognitive function have been reported in patients with Alzheimer's disease and schizophrenia (Bodick et al, 1997; Shekhar et al, 2008). Our results are consistent with these studies. The M2 receptor is predominant in the thalamus (ca 50%) and brainstem (ca 80%), and the other subtype is less than 20% in the thalamus and brainstem (Levey et al, 1991). This means that the mAChR occupancy in the thalamus and brainstem mainly reflects M2 receptor occupancy.
The importance of the M2 receptor in the cortex and hippocampus in working memory has been shown by pharmacological and genetic studies. Pharmacological studies indicate that M2 receptor-preferring antagonists increase acetylcholine (ACh) release and improve cognitive performance in aged rats (Quirion et al, 1995; Rowe et al, 2003; Vannucchi et al, 1997). On the other hand, genetic deletion of M2 receptors impaired working memory (Bainbridge et al, 2008; Seeger et al, 2004; Tzavara et al, 2003). The opposite effects of blockade and genetic deletion of M2 receptor on working memory suggest that the significance of M2 receptor function is to keep an optimal level of cholinergic activity for cognition (Bainbridge et al, 2008). Bainbridge et al (2008) noted that ACh release by M2 receptor antagonists is effective for decreased cholinergic tone. When cholinergic tone is not lowered, the loss of M2 receptors by genetic deletion leads to excess ACh and is detrimental to cognitive function. Our present result extends these findings, and suggests the important role of M2 receptors in the thalamus and brainstem on working memory. This interpretation can be supported by further study using a selective antagonist of M2 receptor and PET radioligand for M2 receptor such as [18F]FP-TZTP (Podruchny et al, 2003) in normal subjects.
One may suggest that ACh release by blocking M2 receptors in the thalamus and brainstem affected [11C](+) 3-MPB binding, because a microdialysis study showed that scopolamine-induced ACh release in the brainstem (Baghdoyan et al, 1998). However, our previous PET study showed that [11C](+)3-MPB did not compete with endogenous ACh (Nishiyama et al, 2001).
Cognitive performance was not impaired 24 and 48 h post-scopolamine. Steeper regression line between the mAChR occupancy and cognitive impairment drawn by the data points of 2 and 6 h than 2, 6, 24, and 48 h indicates the existence of the threshold to induce cognitive impairment in the pooled cortex, thalamus, and brainstem, respectively. This interpretation is consistent with our previous PET study with [11C](+)3-MPB, which showed 30–40% reduction of BPND with the Logan arterial input method in the frontal, temporal, occipital cortices, hippocampus, thalamus, and brainstem in aged monkeys (19.0±3.3 years old) compared with young adult monkeys (5.9±1.8 years old; Tsukada et al, 2000b). Aged monkeys (20.3±2.6 years old) showed an impairment of working memory performance and donepezil, an acetylcholinesterase inhibitor, improved working memory performance (Tsukada et al, 2004). Taken together with this study, scopolamine may occupy mAChRs at a similar or greater level in aged brain and induces cognitive impairment. Further study is needed to investigate the threshold of the mAChR occupancy to induce cognitive impairment in detail.
This study did not allow the use of a large number of monkeys. The limitation of a small sample size was partly balanced with a protocol using each monkey as its own control, thereby eliminating errors related to interindividual variability in mAChR density.
Conclusions
This PET study using [11C](+)3-MPB and a behavioral test using the T-DMS task indicates that 2 h post-administration scopolamine occupies mAChRs in a dose-dependent manner with cognitive impairment. Although cognitive impairment recovered completely within 24 h, mAChR occupancy by scopolamine lasted 24 h post-administration in most brain regions. It took 48 h to recover mAChR occupancy completely. The data indicate that some degree of the mAChR occupancy is needed to induce cognitive impairment, and a major involvement of brainstem mAChR occupancy in working memory.
References
Ahlskog JE, Richelson E, Nelson A, Kelly PJ, Okazaki H, Tyce GM et al (1991). Reduced D2 dopamine and muscarinic cholinergic receptor densities in caudate specimens from fluctuating parkinsonian patients. Ann Neurol 30: 185–191.
Aigner TG, Mishkin M (1986). The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol 45: 81–87.
Alexander GE, Crutcher MD (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271.
Baghdoyan HA, Lydic R, Fleegal MA (1998). M2 muscarinic autoreceptors modulate acetylcholine release in the medial pontine reticular formation. J Pharmacol Exp Ther 286: 1446–1452.
Bainbridge NK, Koselke LR, Jeon J, Bailey KR, Wess J, Crawley JN et al (2008). Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav Brain Res 190: 50–58.
Bartus RT, Johnson HR (1976). Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacol Biochem Behav 5: 39–46.
Bechara A, van der Kooy D (1989). The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci 9: 3400–3409.
Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A et al (1997). Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 54: 465–473.
Broersen LM, Heinsbroek RP, de Bruin JP, Joosten RN, van Hest A, Olivier B (1994). Effects of local application of dopaminergic drugs into the dorsal part of the medial prefrontal cortex of rats in a delayed matching to position task: comparison with local cholinergic blockade. Brain Res 645: 113–122.
Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM (1988). Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia 26: 685–700.
Buccafusco JJ, Jackson WJ, Gattu M, Terry Jr AV (1995). Isoarecolone-induced enhancement of delayed matching to sample performance in monkeys: role of nicotinic receptors. Neuroreport 6: 1223–1227.
Buccafusco JJ, Terry Jr AV, Goren T, Blaugrun E (2003). Potential cognitive actions of (n-propargly-(3r)-aminoindan-5-yl)-ethyl, methyl carbamate (tv3326), a novel neuroprotective agent, as assessed in old rhesus monkeys in their performance of versions of a delayed matching task. Neuroscience 119: 669–678.
Buccafusco JJ, Terry Jr AV, Murdoch PB (2002). A computer-assisted cognitive test battery for aged monkeys. J Mol Neurosci 19: 179–185.
Caccamo A, Fisher A, LaFerla FM (2009). M1 agonists as a potential disease-modifying therapy for Alzheimer's disease. Curr Alzheimer Res 6:112–117.
Chafee MV, Goldman-Rakic PS (1998). Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940.
Collerton D (1986). Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience 19: 1–28.
Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B (2000). Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 48: 381–388.
Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E (2002). Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 7: 1083–1091.
Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H (1991). Learning disturbances following excitotoxic lesion of cholinergic pedunculo-pontine nucleus in the rat. Brain Res 544: 126–132.
Drachman DA (1977). Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology 27: 783–790.
Edginton T, Rusted JM (2003). Separate and combined effects of scopolamine and nicotine on retrieval-induced forgetting. Psychopharmacology 170: 351–357.
Enna SJ, Bird ED, Bennett Jr JP, Bylund DB, Yamamura HI, Iversen LL et al (1976). Huntington's chorea. Changes in neurotransmitter receptors in the brain. N Engl J Med 294: 1305–1309.
Everitt BJ, Robbins TW (1997). Central cholinergic systems and cognition. Annu Rev Psychol 48: 649–684.
Floresco SB, Braaksma DN, Phillips AG (1999). Thalamic–cortical–striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19: 11061–11071.
Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI (1995). Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer's disease. J Neurochem 64: 1888–1891.
Funahashi S, Bruce CJ, Goldman-Rakic PS (1989). Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349.
Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ et al (2005). Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav 81: 575–584.
Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA (2009). Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology 202: 355–369.
Hannula DE, Tranel D, Cohen NJ (2006). The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci 26: 8352–8359.
Haraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (1997). Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies. Drug Metab Dispos 25: 675–684.
Hardman JG, Limbird LE (1996). Goodman and Gilman's the Pharmacological Basis of Therapeutics. McGraw-Hill: New York. . pp 149–150.
Hasselmo ME, Stern CE (2006). Mechanisms underlying working memory for novel information. Trends Cogn Sci 10: 487–493.
Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL (1996). Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res 711: 102–111.
Höhmann C, Antuono P, Coyle JT (1998). Basal forebrain cholinergic neurons and Alzheimer's disease. In: Iversen LL, Iversen SD, Snyder SD (eds). Psychopharmacology of the Aging Nervous System. Plenum Press: New York. . P 69–106.
Honer WG, Prohovnik I, Smith G, Lucas LR (1988). Scopolamine reduces frontal cortex perfusion. J Cereb Blood Flow Metab 8: 635–641.
Hudzik TJ, Wenger GR (1993). Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey. J Pharmacol Exp Ther 265: 120–127.
Inglis WL, Olmstead MC, Robbins TW (2001). Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav Brain Res 123: 117–131.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S (2003). Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci USA 100: 7965–7970.
Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H (2002). Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol 88: 715–731.
Kozak R, Bowman EM, Latimer MP, Rostron CL, Winn P (2005). Excitotoxic lesions of the pedunculopontine tegmental nucleus in rats impair performance on a test of sustained attention. Exp Brain Res 162: 257–264.
Kubo T, Fukuda K, Mikami A, Maeda A, Takahashi H, Mishina M et al (1986). Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature 323: 411–416.
Lange KW, Javoy-Agid F, Agid Y, Jenner P, Marsden CD (1992). Brain muscarinic cholinergic receptors in Huntington's disease. J Neurol 239: 103–104.
Langmead CJ, Watson J, Reavill C (2008). Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 117: 232–243.
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991). Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11: 3218–3226.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996). Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16: 834–840.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ et al (1990). Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10: 740–747.
Matsumura M, Watanabe K, Ohye C (1997). Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res 28: 155–165.
Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1983). Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214: 170–197.
Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1984). Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience 12: 669–686.
Mesulam MM, Mufson EJ, Wainer BH (1986). Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res 367: 301–308.
Mewaldt SP, Ghoneim MM (1979). The effects and interactions of scopolamine, physostigmine and methamphetamine on human memory. Pharmacol Biochem Behav 10: 205–210.
Miller RJ, Hiley CR (1974). Anti-muscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature 248: 596–597.
Mitchell AS, Dalrymple-Alford JC, Christie MA (2002). Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. J Neurosci 22: 1922–1928.
Nishiyama S, Tsukada H, Sato K, Kakiuchi T, Ohba H, Harada N et al (2001). Evaluation of PET ligands (+)N-[11C]ethyl-3-piperidyl benzilate and (+)N-[11C]propyl-3-piperidyl benzilate for muscarinic cholinergic receptors: a PET study with microdialysis in comparison with (+)N-[11C]methyl-3-piperidyl benzilate in the conscious monkey brain. Synapse 40: 159–169.
Ohno M, Yamamoto T, Watanabe S (1992). Effects of intrahippocampal injections of N-methyl-D-aspartate receptor antagonists and scopolamine on working and reference memory assessed in rats by a three-panel runway task. J Pharmacol Exp Ther 263: 943–950.
Owen AM (2000). The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp Brain Res 133: 33–43.
Plakke B, Ng CW, Poremba A (2008). Scopolamine impairs auditory delayed matching-to-sample performance in monkeys. Neurosci Lett 438: 126–130.
Penetar DM, McDonough Jr JH (1983). Effects of cholinergic drugs on delayed match-to-sample performance of rhesus monkeys. Pharmacol Biochem Behav 19: 963–967.
Perry EK (1986). The cholinergic hypothesis—ten years on [review]. Br Med Bull 42: 63–69.
Podruchny TA, Connolly C, Bokde A, Herscovitch P, Eckelman WC, Kiesewetter DO et al (2003). In vivo muscarinic 2 receptor imaging in cognitively normal young and older volunteers. Synapse 48: 39–44.
Potter DD, Pickles CD, Roberts RC, Rugg MD (2000). Scopolamine impairs memory performance and reduces frontal but not parietal visual P3 amplitude. Biol Psychol 52: 37–52.
Prohovnik I, Arnold SE, Smith G, Lucas LR (1997). Physostigmine reversal of scopolamine-induced hypofrontality. J Cereb Blood Flow Metab 17: 220–228.
Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H et al (1995). Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J Neurosci 15: 1455–1462.
Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS et al 2003. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry 160: 118–127.
Rasmusson DD, Dudar JD (1979). Effect of scopolamine on maze learning performance in humans. Experientia 35: 1069–1070.
Reinikainen KJ, Riekkinen PJ, Halonen T, Laakso M (1987). Decreased muscarinic receptor binding in cerebral cortex and hippocampus in Alzheimer's disease. Life Sci 41: 453–461.
Rinne JO, Laakso K, Lönnberg P, Mölsä P, Paljärvi L, Rinne JK et al (1985). Brain muscarinic receptors in senile dementia. Brain Res 336: 19–25.
Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A et al (1997). Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology 134: 95–106.
Rostron CL, Farquhar MJ, Latimer MP, Winn P (2008). The pedunculopontine tegmental nucleus and the nucleus basalis magnocellularis: do both have a role in sustained attention? BMC Neurosci 9: 16.
Rowe WB, O’Donnell JP, Pearson D, Rose GM, Meaney MJ, Quirion R (2003). Long-term effects of BIBN-99, a selective muscarinic M2 receptor antagonist, on improving spatial memory performance in aged cognitively impaired rats. Behav Brain Res 145: 171–178.
Rusted JM, Warburton DM (1988). The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology 96: 145–152.
Sawaguchi T, Goldman-Rakic PS (1991). D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947–950.
Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J et al (2004). M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24: 10117–10127.
Sellin AK, Shad M, Tamminga C (2008). Muscarinic agonists for the treatment of cognition in schizophrenia. CNS Spectr 13: 985–996.
Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C et al (2008). Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165: 1033–1039.
Sitaram N, Weingartner H, Gillin JC (1978). Human serial learning: enhancement with arecholine and choline impairment with scopolamine. Science 201: 274–276.
Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR (2006). Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology 51: 238–250.
Steckler T, Keith AB, Sahgal A (1994). Lesions of the pedunculopontine tegmental nucleus do not alter delayed non-matching to position accuracy. Behav Brain Res 61: 107–112.
Stefurak TL, van der Kooy D (1994). Tegmental pedunculopontine lesions in rats decrease saccharin′s rewarding effects but not its memory-improving effect. Behav Neurosci 108: 972–980.
Supèr H, Spekreijse H, Lamme VA (2001). A neural correlate of working memory in the monkey primary visual cortex. Science 293: 120–124.
Taffe MA, Weed MR, Gold LH (1999). Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res 8: 203–212.
Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH (2002). Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology 160: 253–262.
Takahashi K, Murakami M, Miura S, Iida H, Kanno I, Uemura K (1999). Synthesis and autoradiographic localization of muscarinic cholinergic antagonist (+)N-[11C]methyl-3-piperidyl benzilate as a potent radioligand for positron emission tomography. Appl Radiat Isot 50: 521–525.
Tang Y, Mishkin M, Aigner TG (1997). Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci USA 94: 12667–12669.
Terry Jr AV, Buccafusco JJ (2003). The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 306: 821–827.
Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T (2000). Cholinergic neuronal modulation alters dopamine D2 receptor availability in vivo by regulating receptor affinity induced by facilitated synaptic dopamine turnover: positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci 20: 7067–7073.
Tsukada H, Harada N, Ohba H, Nishiyama S, Kakiuchi T (2001a). Facilitation of dopaminergic neural transmission does not affect [(11)C]SCH23390 binding to the striatal D(1) dopamine receptors, but the facilitation enhances phosphodiesterase type-IV activity through D(1) receptors: PET studies in the conscious monkey brain. Synapse 42: 258–265.
Tsukada H, Kakiuchi T, Nishiyama S, Ohba H, Sato K, Harada N et al (2001b). Age differences in muscarinic cholinergic receptors assayed with (+)N-[(11)C]methyl-3-piperidyl benzilate in the brains of conscious monkeys. Synapse 41: 248–257.
Tsukada H, Nishiyama S, Fukumoto D, Ohba H, Sato K, Kakiuchi T (2004). Effects of acute acetylcholinesterase inhibition on the cerebral cholinergic neuronal system and cognitive function: functional imaging of the conscious monkey brain using animal PET in combination with microdialysis. Synapse 52: 1–10.
Tsukada H, Takahashi K, Miura S, Nishiyama S, Kakiuchi T, Ohba H et al (2001c). Evaluation of novel PET ligands (+)N-[11C]methyl-3-piperidyl benzilate ([11C](+)3-MPB) and its stereoisomer [11C](-)3-MPB for muscarinic cholinergic receptors in the conscious monkey brain: a PET study in comparison with [11C]4-MPB. Synapse 39: 182–192.
Turchi J, Saunders RC, Mishkin M (2005). Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci USA 102: 2158–2161.
Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J et al (2003). Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry 8: 673–679.
Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F (1997). Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience 79: 837–846.
Warren NM, Piggott MA, Perry EK, Burn DJ (2005). Cholinergic systems in progressive supranuclear palsy. Brain 128: 239–249.
Wastek GJ, Yamamura HI (1978). Biochemical characterization of the muscarinic cholinergic receptor in human brain: alterations in Huntington's disease. Mol Pharmacol 14: 768–780.
Watanabe M, Okada H, Shimizu K, Omura T, Yoshikawa E, Kosugi T et al (1997). A high resolution animal PET scanner using compact PS-PMT detectors. IEEE Trans Nucl Sci 44: 1277–1282.
Yamamoto S, Ohba H, Nishiyama S, Takahashi K, Tsukada H (2011). Validation of reference tissue model of PET ligand [11C](+)3-MPB for the muscarinic cholinergic receptor in the living brain of conscious monkey. Synapse (in press).
Zavitsanou K, Katsifis A, Mattner F, Huang XF (2004). Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacogy 29: 619–625.
Acknowledgements
We gratefully acknowledge the technical contributions of Kae Nakamura and Eiji Hoshi. This work was supported by a kakenhi grant-in-aid for Exploratory Research. Selected data from this study were presented, in abstract form, at the Society for Neuroscience Annual Meeting, Chicago, 17–21 October 2009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yamamoto, S., Nishiyama, S., Kawamata, M. et al. Muscarinic Receptor Occupancy and Cognitive Impairment: A PET Study with [11C](+)3-MPB and Scopolamine in Conscious Monkeys. Neuropsychopharmacol 36, 1455–1465 (2011). https://doi.org/10.1038/npp.2011.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.31
Keywords
This article is cited by
-
Methocinnamox (MCAM) antagonizes the behavioral suppressant effects of morphine without impairing delayed matching-to-sample accuracy in rhesus monkeys
Psychopharmacology (2020)
-
Application of cross-species PET imaging to assess neurotransmitter release in brain
Psychopharmacology (2015)
-
An animal PET scanner using flat-panel position-sensitive PMTs
Annals of Nuclear Medicine (2014)